Table 44-1 WHO 2008 Classification of AML | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Table 44-2 FAB Classification of AML | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 44-3 Cytogenetic and Mutational Risk Stratification in AML | ||||||||
|---|---|---|---|---|---|---|---|---|
|
complete remission (CR) rate of 38% was seen compared with 75% in patients with wild-type IDH2.23
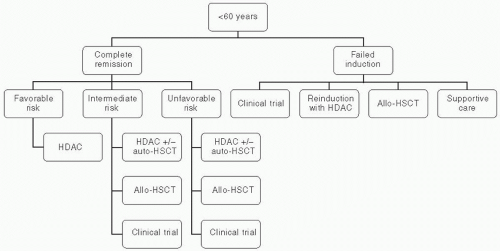
in CR after 5 years.37,38,42,43 In contrast to autologous HSCT, which depends on the effect of a pretransplantation intensive chemotherapy regimen to reduce leukemia cell burden, allogeneic HSCT provides an additional graft-versus-leukemia (GVL) effect that has been shown to decrease relapse rates. The benefits of GVL effect are clearly demonstrated as increased relapse rate of AML patients receiving syngeneic transplants, and the ability of donor lymphocyte infusions to induce partial remissions in patients who relapse after receiving allogeneic HSCT.44,45 Figure 44.1 depicts the treatment algorithm for AML patients younger than 60 years.
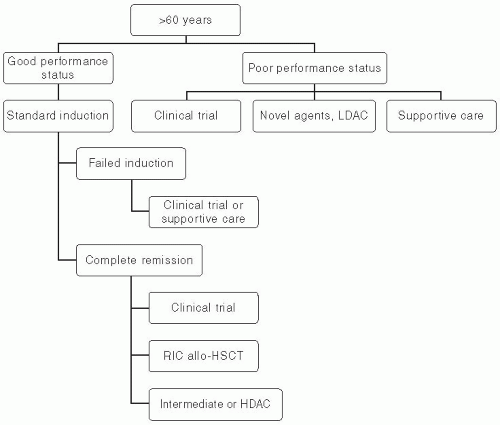
employing RIC allogeneic HSCT showed that development of graft-versus-host-disease (GVHD) was associated with lower rates of relapse.59,60 A large study evaluating the effect of age on the outcomes of RIC allogeneic HSCT in elderly AML patients in first CR revealed no significant effect of age on nontransplant mortality, relapse rates, and disease-free and overall survival. This study supports the notion that available modalities of treatment should be considered thoroughly in elderly patients. Chronologic age alone should not be used as the sole discriminant to deny potentially effective therapy.61 Patients’ preferences, performance status, and comorbidities should be integrated into the medical decision making regarding further postinduction therapy in elderly adults. Figure 44.3 depicts the treatment algorithm for AML patients older than 60 years.
 Figure 44.2 Percentage of different cytogenetic risk groups as a function of age. (From Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481-3485.) |
than 60, or those who are not candidates for intensive therapy.66 Multiple postmarketing reports described severe hepatotoxicity, including hepatic sinusoidal obstruction syndrome (hepatic veno-occlusive disease). Toxicity was noted both among recipients of allogeneic HSCT and those who were treated with gemtuzumab as a single agent.67,68,69 Gemtuzumab was subsequently withdrawn from the market in June 2010.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree