Introduction
When Virchow first described ‘pachymeningitis haemorrhagica interna’, subdural haematoma (SDH) was considered as a fatal disorder. Over the past 150 years, a dramatic improvement has been achieved due to better understanding of its pathophysiology, new imaging methods and refinement of operating techniques. However, the morbidity and mortality are still relatively high, especially in the elderly population.
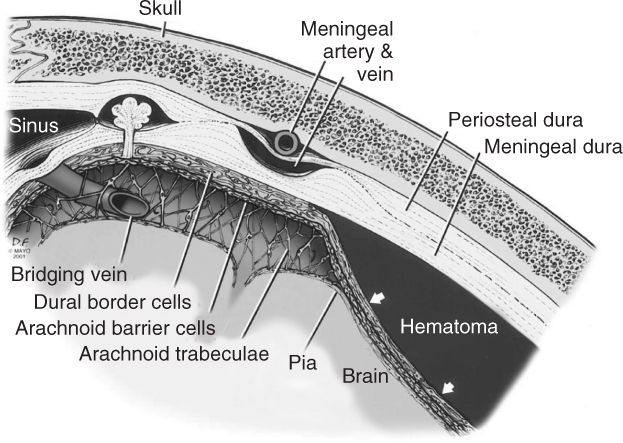
The subdural space lies between the inside of the skull with the periosteal/meningeal dura and the arachnoid layer. That layer contains the cerebrospinal fluid (CSF) and the bridging veins, and surrounds the pia of the brain. This potential subdural space becomes a real subdural space when the dural border cell layer cleaves away from the inner meningeal layer.1 Subsequently a subdural, or more precisely intradural, haematoma may evolve in that space. As the brain atrophies and pulls away from the skull, the strain placed on the meninges is relieved by a splitting open of the weak layer at the dura–arachnoid junction. Figure 60.1 is a magnified schematic representation of a subdural haematoma. The creation of such meningeal spaces in elderly individuals explains, in part, what sometimes appear to be spontaneous haematomas in this area of the meninges in this patient population.
Figure 60.1 Schematic and magnified representation of a subdural haematoma. The dura has two layers: the periosteal dura located on the inner side of the skull and the meningeal dura towards the meninges. Underneath, the arachnoid layer with its trabeculae contains the CSF and the bridging veins. Between the arachnoid and the meningeal dura there is a dural border cell layer. The potential subdural space becomes a real subdural space when the dural border cell layer cleaves away from the inner meningeal layer. Subsequently, a subdural or more precisely intradural haematoma may evolve in that space. (Reproduced from Atkinson et al. Journal of Magnetic Resonance Imaging 2003;17:484–6, with permission from Wiley-Blackwell.)
Haematoma in that space can be either acute or chronic. To fill this potential space and cause pressure on the brain, a mass requires that the pressure applied is greater than the intracranial pressure (ICP), that is, >17 mmHg. Acute SDHs are mainly post-traumatic and of arterial or arteriolar origin. There are two common causes of acute SDH: parenchymal laceration and bridging vessel rupture. Parenchymal laceration represents a severe primary injury and the acute SDH worsens the clinical condition. Surface or bridging vessels might be ruptured during cerebral acceleration–deceleration due to violent head motion. Once the initial bleed has been initiated, the acute SDH becomes subacute and then can turn into chronic SDH. The difference between acute and chronic SDH was defined by McKissock et al. in 1960.2 He denoted ‘acute’ those cases presenting urgent symptoms within 3 days of trauma, ‘subacute’ those presenting within 4–20 days and ‘chronic’ those presenting after 20 days.
In the elderly population, acute SDH has a high morbidity and mortality rate. However, although chronic SDH is common and treacherous, it is potentially treatable. Indeed, the drainage of a chronic SDH is one of the most rewarding and least demanding neurosurgical procedures.
Acute Subdural Haematoma
Acute SDH is a serious, life-threatening condition and its management is complex, especially in the elderly. Acute SDHs are mainly post-traumatic, but can also be spontaneous in the context of coagulopathy. According to McKissock et al.,2 an acute SDH is diagnosed within 1–3 days after trauma. In severe acute SDH, neurological deficit occurs immediately. The mechanism of injury and also the outcome differ between age groups. In younger patients, most acute SDHs are caused by motor vehicle accidents and in older patients by falls.3 It has also been shown that older patients have a higher mortality rate and that it is likely that age causes an alteration in the pathophysiological response of the central nervous system to severe trauma.4 Because of the frequent association of acute SDH with parenchymal injury, surgical management decisions should take into consideration the recommendations for both lesion types. Spontaneous or non-traumatic acute SDHs in the elderly represent a specific clinical entity related mainly to coagulopathy, anticoagulants or antiplatelet therapy. This coagulopathy-associated acute SDH is prevalent in a particular group of patients in which the risk is associated with coagulopathy of one sort or of another.
Pathophysiology
There are various abnormalities predisposing a cortical artery to rupture: avulsion of an arterial twig branching at a right-angle from the parent artery, rupture of a small artery traversing the subdural space and connecting a cortical artery to the dura mater, termed ‘bridging artery’, or adhesions between a cortical artery and the dura mater or arachnoid.5 An acute SDH is related to an arterial bleed in the subdural space. It has been shown that global cerebral blood flow is reduced after acute SDH, leading to a critical ionic imbalance with oedema and widespread cell death. Local pressure causes focal ischaemia, which leads to increased energy metabolism and reduced tissue oxygenation, which can cause infarction a few hours after acute SDH. Even after rapid decompression and haematoma evacuation (<4 h), many patients die. The majority of patients with chronic SDH must, at some time, have had an acute spontaneous SDH. Therefore, probably, many of the so-called spontaneous cases may have been the result of a brisk head movement not severe enough to be considered trauma or of an unrecognized or forgotten trauma. The recognition of spontaneous acute SDH as a clinical entity is important, since many of these patients may subsequently go on to develop chronic SDH.
Epidemiology
Studies conducted after the introduction of computed tomographic (CT) scanning have reported an incidence of acute SDH between 10 and 30% in patients admitted with severe traumatic brain injury. When including mild, moderate and severe head injuries, 11% present with acute SDH.3 More than one-third of cases of acute SDH are in patients over 60 years old. Similarly, about one-third of cases of admitted head injuries in the elderly population will have an acute SDH.6 Only 30–40% of acute SDHs requiring surgery are isolated lesions. In the majority of cases, an acute SDH is associated with other intracranial and extracranial injuries. Associated intra- and extracranial lesions have been reported in larger series to occur in 50% of patients presenting with head trauma and in 70% of patients with Glasgow coma scale (GCS) <10.3
Clinical Presentation
Patients with acute SDH are most of the time comatose: 40–80% of patients had a GCS score of 8 or less.3 A lucid interval has been described in 10–40% of patients before admission but there is no conclusive evidence that this correlates with outcome. Pupillary abnormalities are observed in 30–50% of patients on admission or before surgery. Acute subdural haematoma may be located along the falx, between the two cerebral hemispheres with the ‘falx syndrome’: paresis or focal seizures contralateral to the haematoma.
Investigation
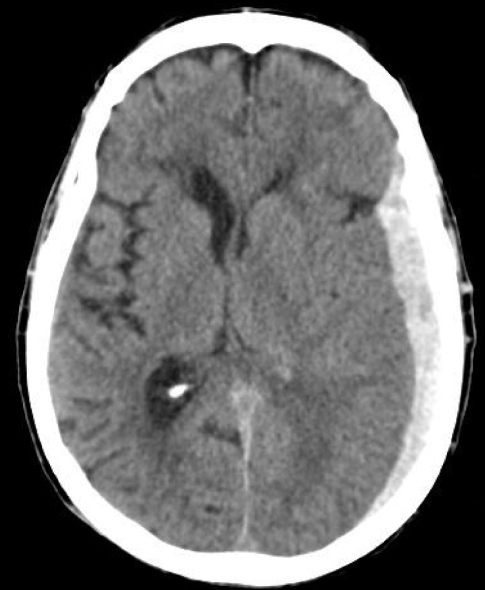
CT scanning is the method of choice. On a CT scan, acute blood collection with a haematocrit >23% has a higher density than the brain parenchyma, that is, >80 Hounsfield units. Acute SDHs are classically hyperdense and crescent-shaped, with a concave surface away from the skull. However, they can have a convex appearance, especially in the early stage of bleeding. Subdural blood can also be seen as a layering density along the tentorium cerebelli and the falx. It is also important to identify the brain parenchyma lesion such as intracerebral haematoma and contusion, subarachnoid haemorrhage, midline shift, brain swelling and skull fractures. An example of a left acute SDH is displayed Figure 60.2.
Figure 60.2 CT scan of a left acute subdural haematoma in an elderly patient. The hyperdensity is crescent-shaped with a concave surface away from the skull. Midline shift is inferior to the thickness of the haematoma.
Management
Observation for a small acute SDH is usual. Surgery should be considered for progressive neurological deterioration. The main difficulty in the elderly population with acute SDH is to decide whether or not an old patient in a coma with an acute SDH should be operated on or not, as the morbidity and the mortality are known to be very high.
Small SDHs often do not require evacuation, as surgery may increase the brain injury if there is a hemispheric swelling with herniation risk through the craniotomy. Patients in a coma, that is, with a GCS score of 3–8, on initial presentation were found to be significantly associated with increased mortality and a poor outcome. Initial pupillary reaction was also shown to be a very important prognostic factor for outcome. For elderly patients with both pupils dilated and non-reactive to light an unfavourable outcome was predicted (death rate almost 100%).3, 7, 8 Concomitant parenchymal brain lesions and traumatic subarachnoid haemorrhage were also predictive of a worse outcome, due to an increased intracranial pressure.9 Studies also showed an increase in mortality with large haematoma; the survival rate dropped below 50% when the acute SDH thickness exceeded 18 mm. A midline shift of more than 10 mm was found also to be correlated with a high mortality. However, it is possible that patients older than 65 years tolerate a greater midline shift because of brain atrophy. The difference between the subdural haematoma thickness and the midline shift seems to be a very strong prognostic factor. In fact, a striking decline in the survival rate was found when the midline shift was greater than the haematoma thickness. When the midline shift was greater than the thickness of the SDH, that is, more brain swelling than SDH, the survival rate was less than 25%.4 It has been suggested that all patients with acute SDH in a coma (GCS score <9) should undergo intracranial pressure (ICP) monitoring. Surgery is indicated only when ICP values are <40 mmHg owing to the poor outcome of elderly patients with high ICP monitoring.3 In patients with acute SDH and indications for surgery, surgical evacuation should be performed as soon as possible,3 but recent studies failed to show any significant difference in outcomes between patients undergoing early (<4 h) or delayed (>4 h) surgery.4 The haematoma is usually evacuated with a craniotomy and subdural drainage or by decompressive craniectomy. A large craniotomy flap is often required to evacuate the thick coagulum and to gain access to possible bleeding sites. The actual bleeding site is often not identified at the time of surgery. Nevertheless, there is no statistically significant difference in the number of re-operations, irrespective of the chosen operative method (large versus simple craniectomy).
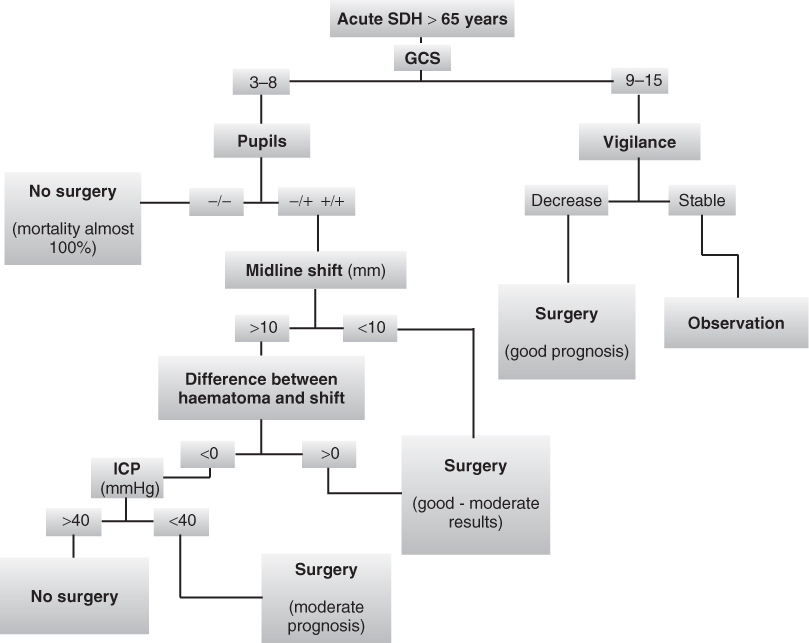
A management algorithm was developed based on clinical and CT parameters to predict the outcome of older patients with acute SDH and to indicate whether surgical haematoma evacuation should be performed.4 This algorithm is represented schematically in Figure 60.3.
Figure 60.3 Acute subdural haematoma management algorithm, based on Petridis et al.4 See the section on management of acute SDH for details.
Trauma and emergency department clinicians encounter an increasing number of older patients with head injuries on premorbid antithrombotic therapies. These patients are at increased risk of acute brain lesions. In the setting of minor head injury, patients may not exhibit signs of overt bleed. Therefore, clinicians must weigh the risks and benefits of completing a head CT to rule out a subclinical haemorrhage. Currently, no studies have addressed the efficacy or utility of reversing anti platelet therapy in traumatic head injury with exogenous platelet administration. However, in warfarin-treated patients presenting with traumatic head injuries, clinicians should have a low threshold for obtaining a head CT to rule out acute intracerebral bleeding. Rapid reversal of anticoagulation to a near-normal profile may be warranted in CT-documented acute haematoma.10 In these patients, the mortality is closely linked to the extension of intracranial bleeding. In patients treated with anticoagulants and suffering from severe head injury, antagonism has to be started as soon as possible after admission. A specific early management protocol for patients exposed to intracranial haemorrhage might reduce the mortality rate.11
Association between mechanical cardiac valves and traumatic head injury is not unusual in the elderly population. There is no significant increase in vital risk if anticoagulants are temporarily stopped for 1–2 weeks. In contrast, mortality and morbidity in antiplatelet drug-treated patients are mainly related to a premorbid condition. In patients presenting with head injuries and acute SDH, prophylactic anticoagulation should be started 72 h after the trauma.12
Outcome
Increasing age is a strong independent factor in prognosis for severe traumatic brain injury, with a significant increase in poor outcome in patients older than 65 years of age. Among patients with acute SDH, there is a relationship between poor outcome and age, low GCS and signs of herniation, but it is not possible to predict death with certainty on the basis of old age and poor GCS.
Chronic Subdural Haematoma
Chronic SDH is a common but puzzling disease. This disease is fairly frequent in the very young but is found mainly in the elderly and those with prior brain atrophy. Although frequent, chronic SDH is treacherous condition: the elderly population with chronic SDH has a demonstrated increased risk of morbidity and mortality.2, 13
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








