The treatment of breast cancer was dominated by radical mastectomy or modified radical mastectomy of the affected breast prior to the 1970s. These included an en bloc removal of the breast, muscles of the chest wall, and contents of the axilla, and at the time they were advocated as the most appropriate local therapy for women with early-stage breast cancers. However, the results of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-06 and other studies found equivalent survival and local control rates among women treated with either mastectomy or lumpectomy followed by whole-breast irradiation (WBI).1,2 The NSABP B-06, which compared mastectomy to lumpectomy with and without radiotherapy in women with invasive carcinoma, found a 39% local recurrence rate at 20 years with lumpectomy alone, which was decreased to 14% with the addition of radiotherapy.1 Several other randomized studies demonstrated equivalent long-term survival and disease-free survival rates in patients treated by breast-conserving therapy (BCT) compared to mastectomy.2-5 Additional randomized studies comparing lumpectomy alone to lumpectomy and radiation clearly demonstrate a 3-fold reduction in local relapse with the use of radiation following breast-conserving surgery.6-10 More recent meta-analyses of trials comparing lumpectomy alone to lumpectomy and radiation demonstrated not only a 3-fold reduction in local relapse, but a small but statistically significant compromise in overall survival with the omission of radiation following lumpectomy.11,12 For patients with ductal carcinoma in situ (DCIS), randomized studies conducted by the NSABP and European Organization for Research and Treatment in Cancer (EORTC) comparing lumpectomy alone to lumpectomy and radiation found a 55% and 43% respective reduction in ipsilateral breast cancer events with the addition of radiotherapy.13,14 From these data, breast-conservation surgery followed by WBI (BCS+RT) became the standard of care for women with stage 0, I, and II breast cancer. BCS+RT involves the surgical removal of the primary tumor, evaluation of the axillary nodes, and local breast irradiation; this treatment is extremely well tolerated with minimal long-term toxicity and favorable cosmetic outcomes.15,16 Despite the obvious cosmetic and potential emotional advantages of BCS+RT, 15% to 30% of patients who undergo lumpectomy do not receive postoperative radiotherapy.17-20 Many patients may choose mastectomy or lumpectomy alone over BCS+RT due to the protracted course of daily treatment involved with WBI, which consists of daily radiotherapy to the whole breast for 25 treatments usually followed by a 5 fraction boost to the tumor bed, all delivered over the course of 6 to 6.5 weeks. Other reasons that steer women away from BCS+RT are physician bias, patient age, fear of radiation treatments, distance from a radiation treatment facility, and socioeconomic factors.18,21-24
Based on the numerous randomized studies noted above, it is standard of care for all women, regardless of age or tumor size, to receive radiotherapy in the setting of BCT to reduce local recurrence. However, in recent years investigators have tried to identify subsets of women who may not benefit from the addition of radiotherapy to lumpectomy for early-stage breast cancer. A prospective study from the Cancer and Leukemia Group B (CALGB) randomized women 70 years of age or older whose tumors were less than 2 cm and estrogen receptor–positive to tamoxifen ± radiotherapy.25 Even though radiotherapy significantly reduced the rate of local recurrence (from 4% to 1%), there was no difference in overall survival, and the investigators concluded that “tamoxifen alone is a reasonable choice for adjuvant treatment in such women.” A Canadian trial, published simultaneously, showed that women over age 50 with early-stage breast cancer demonstrated a local relapse rate of 7.7% with lumpectomy and tamoxifen compared to 0.6% with lumpectomy, tamoxifen, and radiation. Although there was no compromise in survival with the omission of radiation, the study was not sufficiently powered to demonstrate small benefits in survival.
Smith et al, using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, identified 8724 women over age 70 years who met the eligibility criteria for CALGB 9343, and found that radiotherapy not only reduced local recurrence but also reduced the rate of any second breast cancer event and subsequent mastectomy.26 They further identified subgroups, such as women between 70 and 79 years with low comorbidities and those with lobular histology, that derived the greatest benefit from radiotherapy. Of note, in the SEER-Medicare database of women age 70 or greater, only 59% of patients treated with breast-conserving surgery received radiation. Collectively, these data suggest that some older women may safely avoid radiation but that it is also rational for elderly women with long life expectancies and low comorbidities to receive radiotherapy after lumpectomy. Many fail to do so, however, given the prolonged course of therapy, resources needed in travel, and distance to a radiotherapy center.
In response, accelerated partial breast irradiation (APBI) has been increasingly studied over the past 15 years as a viable alternative to WBI. In general, APBI involves treating the surgical cavity with a 1 to 2 cm margin, thus reducing the volume of breast tissue irradiated by up to 50% using various radiotherapeutic methods. Technical approaches of partial breast irradiation include multicatheter interstitial brachytherapy, single lumen balloon catheter brachytherapy, intracavitary multiple lumen catheter brachytherapy, external 3-dimensional conformal external beam radiotherapy (3D-CRT) and intraoperative radiotherapy (IORT). Treatment is typically delivered postoperatively, over a short period of time, using large fraction sizes. Advocates of APBI state that it is a safe and well-tolerated therapy that allows for equivalent cosmetic outcomes while significantly increasing quality of life and allowing for an effective treatment of breast cancer. To date, pilot studies of various APBI techniques have been studied, and large, multicenter randomized and controlled studies are under way comparing APBI to WBI.
In standard BCT, radiotherapy is delivered to the whole breast to eliminate areas of occult multicentric in situ or invasive carcinoma. Additional radiotherapy may be delivered to the tumor bed using a “boost” to eliminate the higher burden of microscopic disease that may have been left in close proximity to the tumor bed after lumpectomy.
Following BCS and WBI, the majority of local relapses occur in close proximity to the tumor bed. When discussing a tumor recurrence in the ipsilateral breast, it is important to note the difference between a true recurrence and the development of a second primary in the irradiated breast. A study from Yale defined a second primary as a recurrence distinctly different from the primary tumor with respect to the histologic subtype, location, or ploidy. In patients treated with BCT with 15-year follow-up data,27,28 patients developed both true recurrences and second primaries at similar rates until approximately 8 years, when true recurrence rates stabilized but second primary rates continued to rise. Recht et al also found that the majority of true recurrences occurred in the first 5 to 10 years but with increasing follow-up, there was a higher incidence of second primary tumors that developed in other quadrants of the breast.29 The 20-year update from Veronesi et al comparing mastectomy to BCT showed a nonsignificant difference between the development of true recurrences or second primaries in the ipsilateral breast (0.63 per 100 woman-years of observation) treated with second compared with the contralateral breast (0.66 per 100 woman-years of observation).2
Several retrospective as well as prospective, randomized studies comparing lumpectomy ± WBI have shown that the majority of tumor recurrences occurred at or near the original tumor bed.30,31 Another study by Veronesi found that failures beyond the lumpectomy cavity occurred in 2.9% of patients, consistent with previously published data of 1.5% and 3.5%.9,32,33 These data suggest that the true benefit of radiotherapy may be to decrease the recurrence of tumor at or near the tumor bed, but may not prevent the development of new, second primary breast cancers that may occur in the irradiated breast.
Given that the majority of true local relapses occur adjacent to the tumor bed, along with available pathologic data demonstrating only minimal microscopic tumor burden more than 1 to 2 cm beyond the primary tumor, a rationale now exists for more localized treatment in selected patients. With APBI, a conformal dose of radiation is delivered to a limited volume of breast in a short period of time. Unfortunately, the majority of data regarding APBI involves single institution, nonrandomized studies with small patient populations. To assess the efficacy of APBI, a large, prospective randomized study is needed. Currently, an intergroup trial (National Surgical Adjuvant Breast and Bowel Project B and Radiation Therapy Oncology Group) is randomizing patients with early-stage breast cancer to WBI versus APBI. Accrual has been brisk and is currently enrolling “high-risk” patients including women less than 50 years of age with DCIS or invasive disease or any patient with node-positive or hormone-negative invasive cancer.
Accurate patient selection for APBI is critical to prevent locoregional failures. To date, the American Brachytherapy Society and the American Society of Breast Surgeons (ASBS) have released similar versions of patient selection criteria for APBI, based mainly on both retrospective and prospective studies (Table 95-1). The American Brachytherapy Society criteria for patient selection include age 50 years or more, tumor upto 3 cm in greatest dimension, invasive ductal histology, negative lymph node status, negative marginal status (defined as “no tumor at ink”), applicator placement within 10 weeks of final lumpectomy procedure, and a postlumpectomy cavity with 1 dimension of at least 3.0 cm.34 Very similar to the American Brachytherapy Society recommendations, the ASBS recommendations include age 45 years or older, tumor upto 3 cm in greatest dimension, in-situ or invasive ductal histology, negative lymph node status, and negative marginal status.
| Series | Age | Tumor Size | Histology | Lymph Nodes | Margins |
|---|---|---|---|---|---|
| American Brachytherapy Society | >50 | ≤3 cm | Invasive ductal carcinoma | Negative (ALND or SLN recommended) | Negative (no tumor at inked margin) |
| American Society of Breast Surgeons | ≤45 | ≤3 cm | Invasive ductal or in situ carcinoma | Negative (ALND or SLN recommended) | Negative |
The NSABP B-39/Radiation Therapy Oncology Group (RTOG) 0413 trial randomizing patients to WBI versus APBI uses the following criteria: age 18 years or more, in situ or invasive ductal or lobular histology, tumor size ≤3 cm, negative margins, a target lumpectomy cavity upto 30% of the breast volume, and if invasive histology, 0 to 3 positive axillary nodes with a minimum of 6 sampled nodes in patients with positive nodes. Given the survival benefit from irradiation of the chest wall and regional nodal groups in patients with positive nodes, the wisdom of including patients with positive nodes as candidates for APBI has been debated.35,36 Other clinicopathologic features such as extensive intraductal component, invasive lobular histology, and lymphovascular invasion also still need to be investigated. The results of the NSABP/RTOG randomized trial, which includes patients with the above noted features, may help to refine selection criteria for patients considering APBI as an alternative to WBI. Until more data are available, it appears that the use of APBI per American Brachytherapy Society or ASBS recommendations represents a reasonable cohort of patients to consider for partial breast irradiation. However, we advise the enrollment of patients for APBI on clinical trials, to help establish selection criteria, document patterns of recurrence, identify acute and long-term toxicities, and develop alternative technical approaches and fractionation strategies.
The goal in APBI is to deliver a homogeneous dose of radiation in a short period of time to the tumor bed with additional margin. This may be achieved using several distinct radiotherapy techniques and include multicatheter interstitial brachytherapy, single lumen balloon catheter brachytherapy, intracavitary multiple lumen catheter brachytherapy, 3D-CRT, and IORT. Each technique is vastly different from the others in terms of degree of invasiveness, radiation delivery, operator proficiency, acceptance among radiation oncologists, and length of treatment. However, each technique is able to deliver a homogeneous dose of radiation to the target area, which in theory is radiobiologically equivalent to conventional protracted WBI with respect to local tumor control, as well as acute and long-term toxicity.
There is a considerable amount of phase I and II data available investigating APBI with similar local control rates as compared to WBI at 5 years. However, most of these data evolved from patients who received multicatheter interstitial breast brachytherapy, which is an intricate, labor-intensive procedure that requires skill on the part of the radiation oncologist. More recently, the intracavitary catheters, such as the MammoSite balloon catheter, external beam radiotherapy, and IORT, have been investigated as alternative methods of APBI.
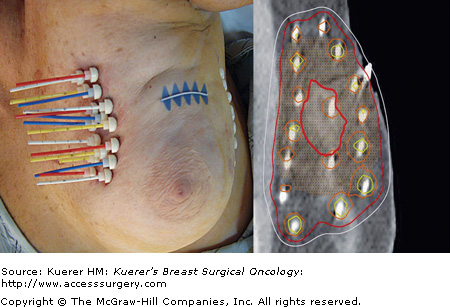
Multicatheter interstitial brachytherapy has been the longest-used APBI technique that originated as a technique for delivering a tumor bed boost following WBI (Table 95-2). Through this approach, flexible afterloading catheters are placed through the breast tissue in several planes, to ensure adequate coverage of the lumpectomy cavity with margin (Fig. 95-1). Generally, these catheters are placed at 1 to 1.5 cm intervals, in several planes, for a total of 10 to 20 catheters to ensure a homogeneous dose covering the target area. Low-activity sources with dose rates in the range of 0.4 to 2 Gy/h are used in low dose rate (LDR) brachytherapy, while high-activity sources with dose rates greater than 12 Gy/h are used in high dose rate (HDR) brachytherapy. Medium dose rate (MDR) and pulsed dose rate (PDR) brachytherapy have also been investigated as alternative methods. With respect to APBI, LDR sources are implanted for approximately 2 to 5 days while the patient is admitted as an inpatient, while HDR brachytherapy allows for an outpatient treatment, fractionated over the course of a week, with a treatment time on the order of seconds to minutes. Implants are carried out using iridium 192 (192Ir) sources of uniform or varying source activities. Remote afterloading with HDR brachytherapy allows for flexibility in treatment planning, given programmable dwell times for each catheter.
| Series | No. of Patients | Dose Rate | APBI Scheme (Dose [Gy] × Fraction No.) | Median FU (mo) | TR/MM (%) | Regional Nodal Failure (%) | Good/Excellent Cosmesis (%) |
|---|---|---|---|---|---|---|---|
| Oschner Clinic 37,38 | 84 | HDR LDR | 4.0 × 8 45 × 1 | 84 | 2.5 | 6 | 75 |
| New Orleans, LA | |||||||
| William Beaumont Hospital39 | 199 | HDR LDR | 4.0 × 8 or 3.4 × 10 | 65 | 2 | 4 | 99 |
| Royal Oaks, MI | 50 × 1 | ||||||
| RTOG 95-1740 | 99 | HDR LDR | 3.4 × 10 45 × 1 | 45 | 3 | 2 | NR |
| NIO I45,46 | 45 | HDR | 4.33 × 7 or | 84 | 0 | NR | 84 |
| Budapest, Hungary | 5.2 × 7 | ||||||
| NIO II47 | 126 | HDR | 5.2 × 7 | 36 | 1.2 | NR | 86 |
| Budapest, Hungary |
The Oschner Clinic in New Orleans, Louisiana, first investigated the use of LDR and HDR interstitial implants following lumpectomy in patients with DCIS or invasive ductal histology, with a tumor size less than 4 cm, negative margins, and 0 to 3 positive axillary nodes.37 They randomized 50 patients in block fashion to either LDR (45 Gy over 3.5-6 days) or HDR (32 Gy over 4 days in 8 fractions). The target volume included the lumpectomy cavity with 2 cm of circumferential breast tissue. In their original report with a median follow-up of 75 months, there was only 1 breast recurrence (2%) and 3 regional nodal failures (6%), with only 1 nodal failure among the 9 patients with positive nodes upon study entry.
In a retrospective, case-control study, King et al identified patients who met the eligibility criteria for the brachytherapy trial but who received WBI.38 They matched these patients to brachytherapy-treated patients according to characteristics based on tumor size, breast size, and pathologic stage. Using this case-control cohort, they found no difference in breast recurrences (2% vs 5%) and locoregional recurrences (8% vs 5%) in patients treated with APBI and WBI, respectively. There was also a nonsignificant difference in cosmesis rated as good or excellent at 20 months between the APBI and WBI groups (75% and 85%, respectively).
The William Beaumont Hospital group has the largest experience using interstitial brachytherapy with the longest reported follow-up of 199 patients with early-stage breast cancer (JNCI).39 Eighty percent of these patients were treated on institutional protocols with the following criteria: invasive ductal histology, tumor size less than 3.0 cm, negative margins (2 mm or more), age more than 40 years, and negative lymph nodes. The other 20% were treated with APBI for “compassionate” reasons and included patients with close margins, DCIS, participation in other studies, and timing of radiotherapy after lumpectomy. The median age was 65 years and 12% of patients had 1 to 3 positive lymph nodes. One hundred twenty patients were treated with LDR brachytherapy, receiving 50 Gy over 96 hours, while the rest of the cohort underwent HDR brachytherapy, receiving either 32 Gy in 8 fractions or 34 Gy over 10 fractions. The target volume included the lumpectomy cavity with a 1 to 2 cm margin for all patients. The group also included a matched pair analysis to compare the rate of local recurrence between APBI and WBI. At 60 months, they reported a 1% local recurrence rate in both the APBI and WBI groups. There was also no difference in distant metastases, disease-free survival, cause-specific survival, or overall survival between the 2 groups. Furthermore, in patients with 60-month follow-up, 99% of patients reported their cosmesis to be good or excellent.
The RTOG conducted the first multi-institutional trial (RTOG 95-17) consisting of interstitial brachytherapy to treat early-stage breast cancer patients.40 This was a phase I/II trial to determine the feasibility, reproducibility, toxicity, cosmesis, local control, and survival of patients treated with lumpectomy and axillary lymph node evaluation followed by APBI using interstitial brachytherapy. One hundred women were enrolled and 99 were found to be eligible. Eighty-seven patients were T1 and 20 patients had 1 to 3 positive lymph nodes. Thirty-three patients were treated using LDR (45 Gy over 4.5 days) and 66 using HDR (34 Gy over 10 fractions in 5 days). With a median follow-up of 3.7 years, 3 patients developed an in-breast recurrence and 3 patients experienced a nodal failure. In a recent update presented at the American Society of Therapeutic Radiation Oncology in November 2006 with a median follow-up of 6 years, 3% and 6% of patients treated with HDR and LDR experienced an in-breast failure, with the majority of failures being classified as a true recurrence/marginal miss.41 The authors concluded that “multicathether partial breast brachytherapy on this trial experienced excellent in-breast control rates.”
There have been several European groups that have investigated the efficacy of multicatheter interstitial brachytherapy to deliver APBI. Many of the early studies were fraught with poor patient selection and outdated treatment planning modalities.42-44 For the purpose of this discussion, we will focus on the more recent European studies, including 1 prospective, randomized controlled study comparing WBI to APBI using interstitial brachytherapy. The National Institute of Oncology (NIO) in Budapest, Hungary, has much experience in the use of HDR interstitial implants to provide APBI.45,46 They treated women of any age with pathologic T1 tumors (in situ carcinoma and invasive lobular carcinoma were excluded) with negative margins and lymph nodes that were pathologically negative (or <2 mm micrometastases). Forty-five patients were treated to a total of 30.3 Gy (n = 8) or 36.4 Gy (n = 37) in 7 fractions over 4 days. The authors included a control group of patients who met the eligibility criteria during the same time period and were treated with WBI. With a median follow-up of 7 years, the actuarial ipsilateral failure rate was reported as 9% (n = 3) in the APBI group and 12% in the WBI group. All patients treated with APBI who experienced a recurrence were subsequently treated with lumpectomy followed by 46 to 50 Gy WBI, providing a 100% mastectomy-free recurrence rate. The NIO then conducted a single institution randomized study between 1998 and 2004 of patients more than 40 years with the same eligibility criteria as described above.47 Two hundred fifty-five patients were randomized to 50 Gy WBI (n = 129) or APBI (n = 126) using HDR multicatheter interstitial brachytherapy (36.4 Gy in 7 fractions over 4 days). Patients who were not suitable for implantation received EBRT using an enface electron field of 50 Gy prescribed to the 80% isodose. With a median follow-up of 3 years, the local recurrence rates were reported as 1.3% and 1.9% for APBI and WBI, respectively (p = .99). There was no difference in cause-specific survival, disease-free survival, and distant metastases-free survival. However, they reported fewer grade 2 to 3 skin side effects in patients treated with APBI as compared to WBI (3% vs 17%, p < .001). At 5 years, the actuarial rate of ipsilateral breast failure was 5.5% and 4.4% in PBI and WBI arms, respectively (p = .65). The long-term cosmetic results being rated as good/excellent were 79% and 59% in the PBI and WBI arms, respectively (p = .001).
The MammoSite balloon brachytherapy device (MammoSite Radiation Therapy System [RTS]; Hologic, Bedford, Massachusetts) was introduced in 2002 and is a form of intracavitary brachytherapy that is simpler in its technique and treatment planning as compared to interstitial brachytherapy (Fig. 95-2). The apparatus consists of a double lumen catheter that is 15 cm in length and 6 mm in diameter. The catheter contains a central lumen that allows for a HDR 192Ir source, and a small adjacent lumen for filling the distally located balloon. This spherical MammoSite balloon catheter is available in 2 sizes when inflated, either 4 to 5 cm or 5 to 6 cm in diameter, for variability in the dimensions of a lumpectomy cavity. An elliptically shaped MammoSite balloon catheter is also available that is a fixed 4 × 6 cm in diameter ellipsoid when inflated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree