Fig. 17.1
Radical mastectomy defect
It was not until the late 1960s that strong evidence became available to prove that a less deforming operation was able to deliver similar rates of survival and local recurrence [10].
The third driving force that affected the change to a less deforming operation was the more frequent diagnosis of less advanced disease that did not require radical surgery. Shortly thereafter, evidence was presented that even less aggressive surgery (lumpectomy) followed by radiation therapy offered similar survival rates to mastectomy. Although conserving the breast, radiation therapy was necessary to provide comparable outcomes. In the United States, this external beam radiation was administered at 6,000 gray, although in Europe the dose was normally 4,500 gray. The number of patients in the United States who choose breast conservation (lumpectomy, followed by radiation therapy) has recently declined. This is in part due to many patients not wanting to undergo adjuvant radiation therapy after lumpectomy, partly due to the time commitment required and partly due to the adverse side effects associated with radiation therapy to the breast. The use of radiation therapy, which in the United States is normally given as 4500 gray with a “boost” of 1500 gray, has produced unfortunate radiation induced deformities in the residual breast (Fig. 17.2).
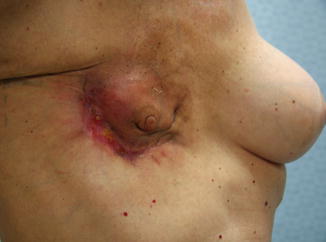

Fig. 17.2
Unusual severe case of radiation mastitis following lumpectomy and radiation therapy
The concept of a skin-sparing mastectomy originated with Freeman in 1962. He suggested a modification of the traditional mastectomy before an implant-based breast reconstruction for benign disease [11]. The skin-sparing mastectomy as a cancer procedure was further described by Toth and Lappert in 1991 [12]. They described preoperative planning of mastectomy incisions to maximize skin preservation and to facilitate breast reconstruction. These modifications in tissue removal have made reconstruction much simpler to achieve. Along with the abandoning of the radical mastectomy in favor of the skin-sparing modified radical mastectomy, other devices were introduced to assist in reconstructing the breast mound.
An important milestone was the development of the silicone breast implant by Cronin (originally for breast augmentation) [13]. The concept of providing a breast mound substitute without a donor site was immediately attractive and popular with patients. Breast augmentation with silicone implants provided far superior results than previous materials, and it seemed possible to use the implants within the mastectomy defect. Unfortunately, postmastectomy reconstruction and augmentation are two very different surgical situations. In the augmentation patient, there is normally ample soft tissue coverage and supple skin. In the mastectomy patient, thinner flaps are the rule and the breast tissue is absent. This results in a lack of adequate soft tissue coverage over the implant, sometimes resulting in significant postoperative complications. These problems included a high rate of capsule contracture, exposure of the implant, deformity from skin rippling over the implant, frequent revision operations, and displaced implants.
The concept of tissue expansion developed by Radovan was an important step. The use of expanders could “recruit” additional soft tissues (skin, fat, and muscle) that attempt to make up for the deficiencies following mastectomy. In fact, the most common use of tissue expanders currently is in breast reconstruction [14]. An effort to improve the soft tissue envelope led to developing procedures that provide “total muscle” coverage of the implants using the serratus as well as the pectoralis muscle. The use of tissue expanders and the use of flaps were utilized to improve the soft tissue envelope. Although these efforts reduced the deformity from skin irregularities and exposure of the implant, the problems with capsule contracture and long-term failure of the reconstruction continued.
Acellular dermal matrix is a biologically altered product that is harvested from cadaveric donors and then processed to remove the cells. It has become popular as an adjunct to soft tissue coverage over the lower third of implants and expanders in cases of immediate breast reconstruction. These materials require no donor site and act as a dermal brassiere to help cover and support the implants. All of these efforts have improved the results of non-autologous reconstruction, still the most common procedure for breast reconstruction undertaken in the United States. Unfortunately, there is still a relatively high failure rate, both short and long term. The FDA post-approval CORE studies of breast implants describe, “between 20–40 % of augmentation patients and 40–70 % of reconstruction patients had re-operations during the first 8–10 years after they received their implants. Although routine replacement is not necessary, many women will need additional surgery to modify, remove, or replace their implants.” Additionally, 17 % of patients in whom implants were used for reconstruction had their implants removed without replacement [15].
Reconstruction with the Patient’s Own Tissue
Because of the problems with implant reconstruction, there has always been interest in using the patient’s own tissue to recreate the breast mound. As early as the turn of the century, the latissimus muscle was described for reconstruction. It was not until Carl Hartrampf popularized the transverse rectus abdominis muscle (TRAM) flap in the 1970s that a procedure was devised to use the patulous abdomen (a very favorable donor site) to replace the missing breast [16]. This procedure was truly revolutionary for its time, with the original case experiences reported by Hartrampf found to be very favorable. It was, however, based on very selective patient criteria, excluding those with a history of smoking, diabetes, obesity, macromastia, and hypertension. Nonetheless, the procedure eventually was being performed more commonly, with less rigorous selection.
When the patient selection was not as rigorous, problems with necrosis of the flap and abdominal wall donor site problems (hernia, bulges, etc.) became evident. There were a number of issues that contributed to these problems. First, the procedure harvested the entire rectus muscle and most of the pre-rectus fascia (Fig. 17.3).
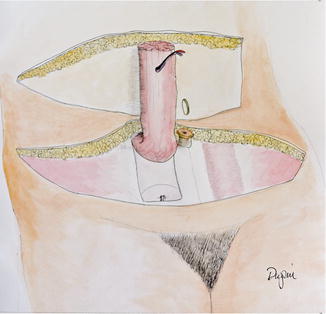

Fig. 17.3
The rotational TRAM flap
This tended to result in functional abdominal wall weakness, more severe for bilateral procedures. The second issue was the circulation to the fat and skin. The primary circulation to the lower abdomen arises from the deep inferior epigastric artery (DIEA). The TRAM flap circulation relied on the deep superior epigastric artery, a continuation of the internal mammary artery. Frequently, the connection of the two vessels around the umbilicus can be tenuous, and without its primary blood supply, ischemia of the flap was a considerable risk. Additionally, in order to move the pedicle flap into the breast defect, a large tunnel had to be created to move the flap (Fig. 17.4). This further impacted the circulation to the upper abdomen.
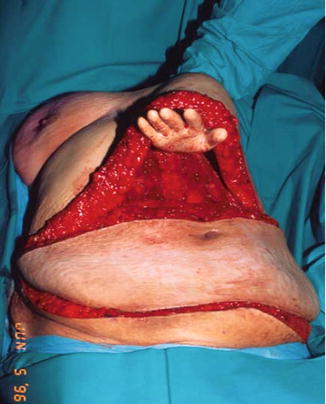

Fig. 17.4
Extensive tunnel required to transfer rotational TRAM flap
Several techniques were proposed to try to improve the reliability, including delay of the flaps and using both rectus muscles.
As a result of these issues, attention was turned to the free transfer of tissue. This procedure would utilize the primary blood supply (DIEA), using the lower abdominal tissue as a free transfer, rather than as a pedicle flap. The free TRAM flap was first reported by Holmstrom in 1979 [17] (Fig. 17.5). This procedure also did not require extensive undermining of the upper abdominal as was necessary in the TRAM flap to pass the flap from the abdomen to the chest.
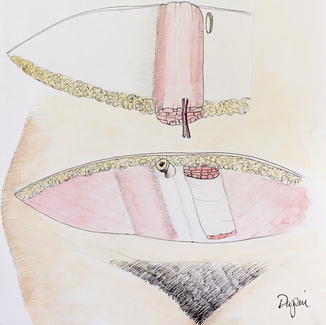

Fig. 17.5
The free TRAM flap
This procedure increased the reliability of the transfer, especially in patients with risk factors such as smoking and obesity. The problem with hernia and bulges in the abdominal wall did not significantly change from the TRAM flap, as the amount of muscle and fascia removed was the same (Fig. 17.6). Lejour and Dome reported a series of patients from whom abdominal flaps were harvested and found significant persistent weakness of the abdominal wall [18].
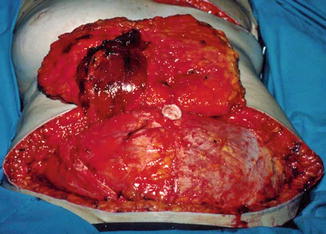

Fig. 17.6
Defect in fascia and muscle in free TRAM
Due to the significant weakening of the abdominal wall, efforts were made to reduce the amount of muscle and fascia harvested. The muscle-sparing TRAM flap was developed that did not remove all of the muscle or fascia (Fig. 17.7). This procedure retains that which is medial or lateral to the perforators arising from the DIEA.
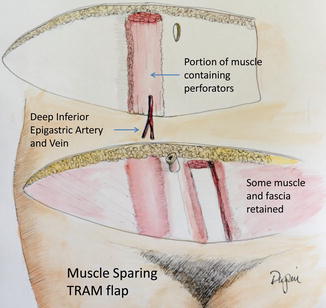

Fig. 17.7
The muscle-sparing TRAM flap
Unfortunately the bulge/hernia rate between the two procedures was found to be about the same. To address this, the use of plastic mesh was advised to reduce the bulge/hernia rate for these procedures. Although the rate of hernia/bulge is improved by mesh, there are attendant problems with its use and there does not appear to be any improvement in the muscle function [19, 20].
In an effort to reduce abdominal wall complications, procedures to harvest the skin and fat without the rectus muscle or fascia were developed. The most common is the deep inferior epigastric artery perforator (DIEP) flap. This free flap is based on vessels that emerge from the deep inferior epigastric artery, enter and pass through the muscle and fascia, and provide blood supply to the overlying skin (perforating vessels) (Fig. 17.8).
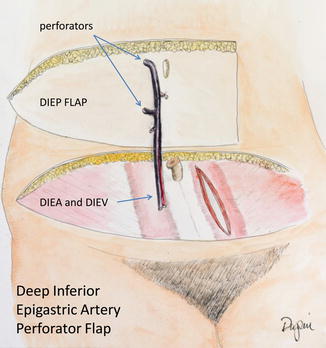

Fig. 17.8
The DIEP flap
First described by Allen and Blondeel [21, 22], the use of the DIEP flap has increased dramatically as a reconstructive option for autologous breast reconstruction. The very low rate of hernia or bulges without the need for mesh repair was a tremendous improvement, despite the somewhat longer operative time. Even bilateral DIEP flaps can be repaired primarily with no tension on the fascia [23].
Once the perforator concept was described, other sources of tissue for reconstruction were rapidly developed based on the concept of “perforator flaps.” These include flaps from the buttocks, thighs, and other areas where there is sufficient fat and skin to harvest. The buttock flaps include those based on perforators arising from the superficial gluteal artery (S-GAP) [24] (Fig. 17.9) and the inferior gluteal artery (I-GAP) [25].
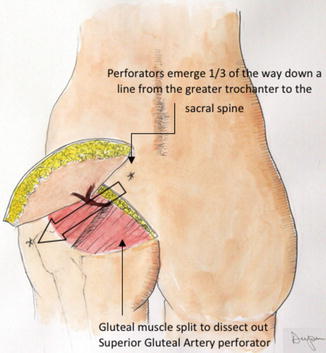

Fig. 17.9
The superior gluteal artery perforator flap
The thigh flaps include the anterolateral thigh flap [26], the gracilis myocutaneous flap [27], and, more recently, a flap based on the second perforating branch of the profunda femoris (Fig. 17.10) [28].
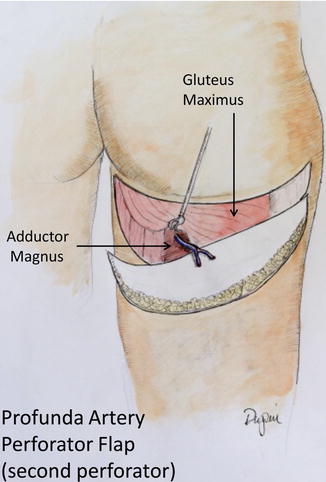

Fig. 17.10
The profunda artery perforator flap
These flaps are typically used in patients when the DIEP flap cannot be used. Patients with a previous abdominoplasty, for example, cannot have the DIEP flap transferred, as all of the perforators to the lower abdomen have been destroyed.
Current Choices
Therefore, two distinct approaches to reconstruction are currently used. The most common approach is expander/implant reconstruction. The advantages are a relatively straightforward operative procedure, a reduced initial morbidity and hospitalization, and the lack of a donor site. The disadvantages include implant-based issues such as capsule contracture, failure of the implant, multiple procedures, and lack of adequate soft tissue coverage (see above).
The most favorable patients are those with relatively small, non-ptotic breasts, especially in bilateral reconstructions. The least favorable are patients with large ptotic breasts, delayed reconstruction patients, and patients who have undergone, or will undergo, radiation therapy. Implant/expander procedures are also less satisfactory in obese patients and patients with macromastia or severe ptosis. The rate of complications, while initially low, also tends to increase over time. Revision procedures have a higher complication rate than the original procedure. Nonetheless, the majority of these procedures are successful and have a high level of patient satisfaction [29].
The second approach to breast reconstruction is autologous in nature, utilizing the patient’s own tissue. The advantages are as follows: permanence, lack of implant problems, ease of reshaping the breast mound, and (for most patients) a tight, flat postoperative abdomen. The disadvantages are higher early complication rate (including the loss of the flap), donor site problems, longer initial operative time, and scars in the donor site area. The rate of total flap loss in microsurgical breast reconstruction is 5 % or less in most series. Patient satisfaction with the procedure and overall cosmetic outcome is quite high [30].
The optimal candidates are patients with delayed reconstruction (especially with radiation changes), patients with poor quality chest wall soft tissues, patients with macromastia or ptosis, and patients with bilateral reconstruction. Patients with extensive disease may require chest wall reconstruction and benefit from free transfers. Poor candidates are patients who cannot withstand anesthesia risks of long surgery, hypercoagulable patients, morbidly obese patients, and smokers.
Other factors that have tended to increase the rate of breast reconstruction include patient demand for mastectomy instead of conservative breast treatment and the discovery of genetic predisposition (BRCA1 and BRCA2) for breast cancer. Despite the original optimism for breast conservation surgery, a significant number of patients continue to select mastectomy as their desired surgical option, with a growing number further opting to undergoing prophylactic mastectomy of the contralateral breast with immediate reconstruction. Although the data is clear that the statistical chance of survival is based upon the original cancer, many patients will still fear a recurrence, no matter how small or insignificant the risk.
The discovery of genetic factors [BRCA gene mutations] responsible for the development of breast cancer has resulted in a new population of patients that requires therapy. Many of these patients, especially those who have seen close family members struggling with breast cancer, opt for bilateral mastectomy, additionally wanting immediate reconstruction.
Immediate Versus Delayed Breast Reconstruction
In the subset of breast cancer patients who require or choose mastectomy, the discussion with the patient focuses upon whether to proceed immediately with reconstruction or to wait a period of time for a delayed procedure. There are sound oncologic reasons for delaying reconstruction in some patients. Patients who will require radiation therapy are more likely to have a much better outcome with delayed reconstruction, especially when autologous reconstruction is performed. A full course of chest wall radiation can result in significant and sometimes unpredictable changes within the radiated flap, including fat necrosis, scarring, and loss of tissue volume. The aesthetic outcomes are far better with delayed reconstruction in these patients. Patients treated with expanders and implants have a significantly higher complication rate for all complications when radiation therapy is used.
Immediate reconstruction provides several benefits. The preservation of the breast skin envelope around the reconstructed breast produces a more aesthetically pleasing result and reduces the visible scar. Immediate reconstruction also avoids the emotional distress of awakening without a breast mound. Immediate reconstruction was noted by Rozen to have a positive effect on anxiety, depression, self-image, and emotional as well as sexual function [31]. There is no evidence that forcing the patient to wait and live with her defect improves patient acceptance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree