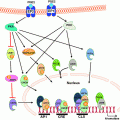
Fig. 5.1
Targeting obesity-related estrogen biosynthesis. Weight loss, anti-diabetics, aromatase inhibitors and a number of other therapies have been shown to decrease estrogen biosynthesis and circulating estrogens, and hence, may prove useful in the treatment of obesity-related postmenopausal breast cancer
5.2.2 Insulin Sensitizers and Anti-diabetics
The commonly used anti-diabetic drug metformin has received much attention in the past decade for its potential as a cancer therapeutic. This stems largely from observational studies demonstrating that metformin use in diabetics is associated with a significant decrease in the risk of developing a number of cancers, including that of the breast [199, 200]. Use of metformin in the neo-adjuvant setting is associated with a significant decrease in breast tumor proliferation as well as an increase in tumor cell apoptosis. A study by Niraula et al. demonstrated that 500 mg three times/day given to women after their diagnostic biopsy for a medium of 18 days caused a 3 % reduction in Ki67 staining and an almost twofold increase in TUNEL staining [201]. In this study, use of metformin was also associated with a 0.5 kg/m2 reduction in BMI. Metformin action appears to be mediated by causing changes in ATP levels within cells. This occurs via a number of converging mechanisms including inhibition of complex I in the mitochondrial electron transport chain [reviewed in 202]. The net effect is a lowering of ATP, leading to an increased ratio of AMP to ATP, known to stimulate AMPK. As a consequence of this observation, the use of metformin in settings where AMPK is important, including metabolism, cell proliferation and estrogen biosynthesis, has been explored. Indeed, metformin causes the inhibition of proliferation of a number of endocrine-related cancer cells, including that of the breast [203–206]. In MCF-7 breast cancer cells, treatment with metformin leads to the regulation of a number of genes responsible for cell cycle arrest, including p27Kip1 and p21Cip1 [204]. Metformin has also been shown to inhibit mTOR and as a consequence, decrease translation initiation and protein synthesis [207]. The effect of metformin on cancer cell growth has also been shown to be dependent on the presence of LKB1 as LKB1-deficient cell lines do not respond to metformin treatment.
The effect of metformin on aromatase expression in breast adipose has also recently been described (Fig. 5.1). It was demonstrated in isolated human breast stromal cells, that consistent with its role to activate AMPK in other tissues, metformin stimulated AMPK and caused the cytoplasmic sequestration of CRTC2 in breast stromal cells at micromolar concentrations [208]. This study also provided a novel mechanism for AMPK activation, namely, via the increased expression of LKB1. A subsequent study demonstrated that metformin acted specifically on aromatase promoter I.3/II, with no effect on promoter I.4 [209]. This suggests that metformin may be beneficial in the treatment of hormone receptor positive breast tumors without the side-effects associated with current endocrine therapy use.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree