6 Hepatocellular Carcinoma: Radioembolization
6.1 Introduction
6.1.1 Hepatocellular Carcinoma Overview
Hepatocellular carcinoma (HCC) is the sixth most common cancer in the world and the third leading cause of cancer mortality worldwide. Although HCC occurs more commonly in regions with a higher incidence of hepatitis, such as in Asia Pacific and Southern Europe, the National Cancer Institute (NCI) and the American Cancer Society (ACS) report the age-standardized incidence rate of HCC at 2.1 per 100,000 population in North America. Both the incidence and mortality rate related to HCC in the United States continue to rise with an estimated 30,640 new cases of HCC diagnosed in 2013 and 21,670 new deaths related to HCC. 1
Risk factors for the development of HCC include viral hepatitis, alcohol abuse, nonalcoholic steatohepatitis (NASH), intake of aflatoxin-contaminated food, diabetes, obesity, and certain hereditary conditions, such as hemochromatosis. The main viruses associated with the development of HCC are the hepatitis B virus (HBV) and hepatitis C virus (HCV); indeed, 50% of HCC cases worldwide are associated with HBV infection, with a further 25% associated with HCV. 2 The end stage of chronic inflammation in the liver, as best characterized by chronic viral infections, is hepatic cirrhosis. This is characterized by a decrease in hepatocyte proliferation and an increase in parenchymal fibrosis, which ultimately results in compromised hepatic function. In the progression of chronic inflammation to fibrosis, there is an upregulation of mitogenic pathways due to increased levels of cytokines (i.e., tumor growth factor, TGF-β1) and reactive oxygen species (ROS) released from damaged liver parenchymal cells, which lead to the propagation of monoclonal populations. 3 These clonal nests of hepatocytes contain telomerase erosions and chromosome aberrations, which limit the proliferative, and hence regenerative, capacity of the liver. 4 , 5 The altered gene expression in these dysplastic hepatocytes predisposes them to becoming premalignant, and, over time, developing into HCC. Because HBV and HCV can also cause HCC independent of cirrhosis, other pathogenic mechanisms have also been postulated, including the integration of HBV DNA into the host genome (where it can cause gene deletions, rearrangements, and chromosome instability) and the synthesis of several oncogenic proteins by HCV. 6
6.2 Radiobiology, Mechanisms of Action and General Approach
6.2.2 Basic Principles
Selective internal radiation therapy (SIRT), also known as radioembolization and/or radiomicrosphere therapy in its current commercial form, uses yttrium-90 (90Y) as a form of brachytherapy whereby microspheres loaded with 90Y are permanently implanted in the target tumor vascular bed within the liver via intra-arterial administration. SIRT aims to selectively deliver radiation to liver tumors while limiting the dose delivered to normal liver parenchyma through the exploitation of the phenomenon of neovascularization of hepatic primary tumors originating exclusively via the hepatic arterial supply. This process allows for the deposition of 90Y microspheres to preferentially implant within liver tumors in a 3 to 20:1 ratio, as compared to the normal liver parenchyma. 7 Although conventional external beam whole liver radiotherapy treatment is not well tolerated, radiotherapy to smaller portions of the liver is well tolerated without significant complications, provided a sufficient amount of normal liver parenchyma is spared. 8 Treatment with 90Y can be currently delivered either as a resin (SIR-Spheres, Sirtex Medical Inc., Sydney, Australia) or as a glass microsphere (TheraSphere, BTG Ltd, Ottawa, Canada). As 90Y decays, it emits high-energy β-radiation (0.97 mEv), which travels a mean of 2.5 mm from the physical microsphere. A single microparticle does not significantly contribute to the overall response to treatment; it is the collective effect of multiple microparticles that creates a cumulative isodose cloud of lethal radiation exposure (via crossfire) in the range of 100 to 1,000 gray (Gy) for a limited time (90Y decays to zirconium-90 [90Z] with a half-life of 64.2 h) 9 ( Fig. 6.1 ). The microparticle size used for SIRT is on average 20 to 60 µm, which is small enough for the particles to enter the tumor vascular network, but large enough to prevent arteriovenous shunting (in most situations). 10
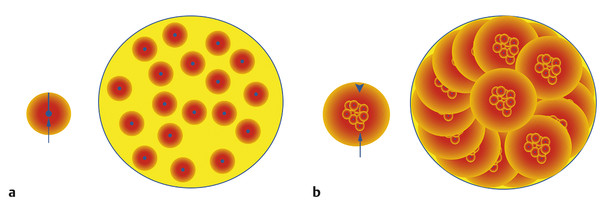
6.2.3 Mechanism of Action of Selective Internal Radiation Therapy
SIRT’s mechanism of action is fundamentally different from that of chemoembolization; in fact, the term radio embolization is a misnomer because no significant effect or response has been demonstrated by the physical particle itself, even when angiographic embolization of nonradioactive microspheres has been performed. 11 Chemoembolization relies on the occlusion of vessels of the tumor, thereby creating stasis and hence maximum exposure of the chemotherapeutic agent to the ischemic environment created within the tumor. In contrast, blood flow and oxygen are essential for SIRT to allow ROS generation via the ionization of water molecules from the emitted β-radiation. This, in turn, results in damage to DNA and activation of apoptosis via an increase in the ratio of BAX:BCL-2 gene expression. 12 Furthermore, cancer cells are more susceptible to oxidative stress due to their relatively low concentration of superoxide dismutase (SOD) and their increased production of superoxide (●O2 −) from their higher aerobic metabolism. 13 SOD is an antioxidant that quenches ●O2 – before it can react with hydroxyl radicals (OH•) to produce hydrogen peroxide (H2O2). Direct double-strand DNA breaks also account for a significant proportion of cellular death. As such, the resin and glass microspheres used for SIRT are small enough to have little or no embolic effect on the medium- to small-sized hepatic arteries, thereby maintaining adequate tissue oxygenation. 11
6.3 Indications for Radioembolization
6.3.4 Staging Systems Used in Chronic Liver Disease
The severity of any underlying chronic liver disease can be assessed using two scoring systems, independent of whether or not the patient has HCC.
The Child–Pugh score is used to assess both the prognosis of underlying chronic liver disease (i.e., cirrhosis) and the necessity of treatment of liver transplantation ( Table 6.1 ). Based on the patient’s serum bilirubin, serum albumin, and international normalized ratio (INR), and the clinical presence of ascites or hepatic encephalopathy, patients are scored from 5 to 15 points based on a linear regression model. Depending on the score, patients are then placed in a class A to C, which reflects their likely mortality over the next year. Class A = 5–6 points with a 100% 1-year survival, class B = 7 to 9 points with an 81% 1-year survival, and class C = 10 to 15 points with a 45% 1-year survival.
Variable | 1 point | 2 points | 3 points |
Total bilirubin, μmol/L (mg/dL) | < 34 (< 2) | 34–50 (2–3) | > 50 (> 3) |
Serum albumin, g/dL | > 3.5 | 2.8–3.5 | < 2.8 |
International normalized ratio | < 1.70 | 1.71–2.30 | > 2.30 |
Ascites | None | Mild | Moderate to severe |
Hepatic encephalopathy | None | Grade I–II | Grade III–IV |
The second scoring system used to assess the severity of chronic liver disease is the Model for End-Stage Liver Disease (MELD) score, which is based on the following equation:
Equation 6.1
This system was initially used to predict the 3-month mortality following a transjugular intrahepatic portosystemic shunt (TIPS) procedure but was also found to be useful for prioritizing recipients of a liver transplant. In hospitalized patients, the 3-month mortality is based on a patient’s MELD score ( Table 6.2 ).
MELD score | Mortality (%) |
> 40 | 71.3 |
30–39 | 52.6 |
20–29 | 19.6 |
10–19 | 6 |
< 9 | 1.9 |
6.4 The Barcelona Clinic Liver Cancer Staging System for HCC
There are several staging systems used to stratify patients with HCC to guide appropriate primary therapeutic treatment and adjuvant therapy, to estimate survival prognosis, and to exchange information without ambiguity. 14 Because patients with HCC often have underlying cirrhosis, the severity of the underlying liver disease, the patient’s functional status, and the extent of the HCC neoplasm require consideration. The most widely accepted model is the Barcelona Clinic Liver Cancer (BCLC) staging system, which has been shown to be best for guiding treatment in early-stage disease, especially in identifying when there is potential benefit from curative therapies. The BCLC classification was formed from several cohort studies and randomized clinical trials conducted by the Barcelona group; the classification uses variables related to tumor stage, liver functional status, physical status, and cancer-related symptoms and links the stage of the disease to a specific treatment strategy via an algorithm ( Fig. 6.2 ). 4
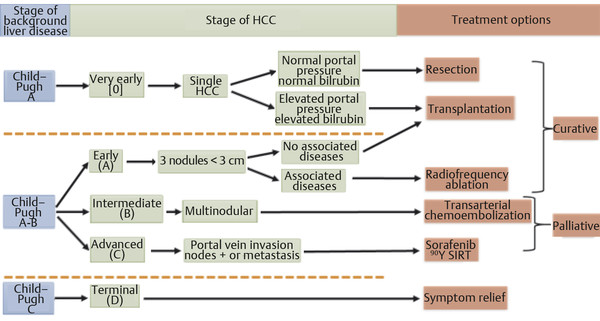
6.5 Treatment Selection for Patients with HCC
The treatment selection for HCC depends on the tumor size, morphology (e.g., portal vein thrombus), and location, as well as the presence of comorbidities (including the extent of the underlying hepatic reserve) and the presence or absence of extrahepatic disease. Historically, intermediate-stage patients (stage B) have been offered conventional Lipiodol-based (Guerbet, Paris, France) chemoembolization. In this population therapeutic intent is balanced between the preservation of hepatic function (against the background of cirrhosis) and the progression of tumor, because both factors ultimately contribute to overall survival. By conventional BCLC criteria, patients with advanced disease (stage C, which includes a myriad of clinical conditions, including portal vein invasion, lower performance status, and extrahepatic metastatic disease) who are considered to have no locoregional option may be relegated to a palliative therapeutic option using sorafenib (Nexavar, Bayer Pharmaceuticals). Finally, in patients with end-stage disease (stage D, Bayer Pharmaceuticals, Leverkusen, Germany), only palliative treatment is offered because the terminal stages of liver decompensation and tumor progression preclude any meaningful benefit. 15 , 16
As more advanced techniques and targeted therapies develop, their evaluation with respect to advanced disease states, quality of life, side-effect/toxicity profile, and depth of response warrants further consideration. Specifically, the role of radioembolization or SIRT within the context of these factors has resulted in hybridization of the BCLC staging system, leading to the incorporation of this less toxic and potentially more effective locoregional therapy within the treatment algorithm. From the BCLC classification, patients with HCC who are candidates for transarterial chemoembolization (TACE) include stage A patients with nonablatable and nonresectable disease, and stage B patients with either unilobar disease or those with a limited number of nodules (1–5). In these patients, the main reason for considering SIRT over TACE includes a better treatment-related quality of life (i.e., less postembolization syndrome and no inpatient stay following treatment) and increased depth of radiographic response; however, no clear survival advantage has been demonstrated in this population. 17 The advantages of SIRT become clearer in the following specific situations (that also include subcategories of BCLC C patients): (1) poor candidates for TACE—stage B patients with bilobar disease or those with multiple tumors (> 5), (2) cases where TACE has previously failed; (3) cases where TACE is contraindicated (e.g., those with portal vein thrombosis), (4) elderly populations, (5) poor Eastern Cooperative Oncology Group (ECOG) performance status (≤ 2), and (6) patients who are under consideration for downstaging to surgical resection or transplantation ( Table 6.3 ).
Lead Author/Year | n | Treatment | Cohort | Median survival (months), | p-value | |||||||||
Whole cohort | BCLC stage A | BCLC stage B | BCLC stage B/C | BCLC stage C | |||||||||
Comparative studies | |||||||||||||
Gramenzi 2014 69 | 63 | SIR-Spheres | BCLC A/B/C | 13.2 mo | 0.96 | 22.1 mo | 0.88 | 6.0 mo | 0.88 | ||||
74 | Sorafenib | BCLC A/B/C | 14.4 mo | 20.4 mo | 8.4 mo | ||||||||
Moreno-Luna 2013 70 | 61 | TheraSphere | BCLC A/B/C | 15 mo | 0.47 | 23.9 mo | 0.40 | 16.8 mo | 0.16 | 8.4 mo | 0.47 | ||
55 | cTACE | BCLC A/B/C | 14.4 mo | 18.6 mo | 13 mo | 10.1 mo | |||||||
Iñarrairaegui 2012 71 | 6 | SIR-Spheres > radical therapy | UNOS T3 uncensored | Not reached | nr | ||||||||
15 | SIR-Spheres | UNOS T3 | 22 mo | ||||||||||
Salem 2011 17 | 123 | TheraSphere | BCLC A/B/C | 20.5 mo | 0.23 | 27.3 mo | 0.74 | 17.2 mo | 0.42 | 22.1 mo | 0.04 | ||
122 | cTACE | BCLC A/B/C | 17.4 mo | 45.4 mo | 17.5 mo | 9.3 mo | |||||||
Lance 2011 72 | 38 | SIR-Spheres | NR | 8.0 mo | 0.33 | ||||||||
35 | cTACE | NR | 10.3 mo | ||||||||||
Kooby 2010 73 | 27 | SIR-Spheres | Okuda I–III | 6 mo | 0.74 | ||||||||
44 | cTACE | Okuda I–III | 6 mo | ||||||||||
Carr 2010 74 | 99 | TheraSphere | NR | 11.5 mo | < 0.05 | ||||||||
691 | cTACE | NR | 8.5 mo | ||||||||||
D’Avola 2009 75 | 35 | SIR-Spheres | First-line | 16 mo | < 0.001 | 16 mo | < 0.001 | ||||||
43 | Std Tx or BSC | First-line | 8 mo | 8 mo | |||||||||
Lewandowski 2009 76 | 43 | TheraSphere | UNOS T3 | 35.7 mo | 0.18 | 35.7 mo | 0.18 | ||||||
43 | cTACE | Censored | 18.7 mo | 18.7 mo | |||||||||
43 | TheraSphere | UNOS T3 | 41.6 mo | 0.008 | 41.6 mo | 0.008 | |||||||
43 | cTACE | Uncensored | 19.2 mo | 19.2 mo | |||||||||
Woodall 2009 77 | 20 | 90Y glass m/s | No PVT | 13.9 mo | 0.01 | 13.9 mo | |||||||
15 | 90Y glass m/s | BCLC C + PVT | 2.7 mo | 2.7 mo | |||||||||
17 | Screen failures | ± PVT | 5.2 mo | 5.2 mo | |||||||||
Goin 2005 21 | 34 | TheraSphere | Okuda I/II | 12.4 mo | NR | ||||||||
38 | cTACE | Okuda I/II | 11.3 mo | ||||||||||
Noncomparative studies | |||||||||||||
Chow 2014 78 | 29 | SIR-Spheres + sorafenib | BCLC B/C | 20.3 mo | 8.6 mo | ||||||||
Khor 2014 79 | 103 | SIR-Spheres | BCLC A/B/C | 23.8 mo | 11.8 mo | ||||||||
Mazzaferro 2013 80 | 52 | TheraSphere | BCLC B/C | 15 mo | 18 mo | 13 mo | |||||||
Sangro 2011 23 | 325 | SIR-Spheres | BCLC A/B/C | 12.8 mo | 24.4 mo | 16.9 mo | 10 mo | ||||||
Salem 2010 81 | 291 | TheraSphere | BCLC A/B/C | NR | 26.9 mo | 17.2 mo | 7.3-EHD/5.4+EHD mo | ||||||
Hilgard 2010 82 | 108 | TheraSphere | BCLC B/C | 16.4 mo | 16.4 mo | Not reached | |||||||
Iñarrairaegui 2010 83 | 25 | SIR-Spheres | BCLC C + PVT | 10 mo | 10 mo | ||||||||
Iñarrairaegui 2010 84 | 72 | SIR-Spheres | BCLC A/B/C | 13 mo | |||||||||
Kulik 2008 85 | 71 | TheraSphere | NR | 15.4 mo | |||||||||
25 | BCLC | C branch PVT | 10 mo | 10 mo | |||||||||
12 | BCLC | C main PVT | 4.4 mo | 4.4 mo | |||||||||
Salem 2005 86 | 43 | TheraSphere | Okuda I/II | ||||||||||
Child–Pugh A | 20.5 mo | ||||||||||||
Child–Pugh B | 13.8 mo | ||||||||||||
Abbreviations: BCLC, Barcelona Clinic Liver Cancer staging system; mo, months; B/C, ??; cTACE, conventional transarterial chemoembolization; NR, not recorded; Std Tx, standard therapy; BSC, best supportive care; PVT, portal vein thrombosis; −EHD/+EHD: without or with extrahepatic disease. | |||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree