7 Colorectal Hepatic Metastatic Disease: Ablation
7.1 Introduction
Percutaneous image-guided tumor ablation can be used to effectively treat hepatic metastases from primary colorectal cancer. Specific advantages of percutaneous tumor ablation include the ability to treat patients with limited disease who are not candidates for surgical resection based on medical comorbidities, generally reduced morbidity and mortality compared to surgical therapies, relative cost-effectiveness, and equivalent or near-equivalent long-term outcomes in well-selected patient populations. This chapter reviews how to perform percutaneous ablation, specifically focusing on case selection, patient evaluation, technical considerations in performing ablation, potential complications, and clinical outcomes.
7.2 Clinical Indications
Percutaneous image-guided thermal ablation is commonly indicated for the treatment of liver-dominant or limited metastatic disease from primary colorectal cancer. Use of thermal ablation for colorectal liver metastases has the best long-term outcomes in patients who have undergone resection of their primary colorectal tumor and have liver-only metastases, and when liver metastases are small and few in number (< 3 cm diameter, and < 3 in number). 1 However, thermal ablation can be offered in greater disease burden (tumors > 3 cm or > 3 in number) in select cases. Patients must be evaluated in the context of their disease burden as a whole, and a comprehensive treatment plan developed that considers timing of resection of the primary cancer, use of adjuvant (and neoadjuvant) chemotherapy, staged hepatic resection combined with ablation, and treatment of other potential sites of metastases (e.g., lung metastases) either with additional ablation or surgery. Thus indications for thermal ablation can be broadly divided into (1) limited liver-only metastases with thermal ablation as the primary and singular treatment (i.e., curative intent) in situations where tumors are unresectable, patients are medically inoperable or lack sufficient hepatic reserve, or patients prefer this option; and (2) more extensive metastatic disease with thermal ablation as one of several treatments to control disease in combination with systemic therapy, additional locoregional treatment (transarterial therapies or stereotactic radiation), or extensive or staged surgical resection. Ultimately, the overall intent of treatment should be curative (i.e., treatment of all visible sites of metastases) because the benefit of palliative treatment of some metastases (either through ablation of some metastases or through partial/incomplete ablation of large metastases) has not been proven.
7.3 Contraindications
Contraindications to ablation are largely relative, according to patient and tumor characteristics. Patient characteristics that might preclude ablation include very poor performance status (e.g., Eastern Cooperative Oncology Group (ECOG) performance status ≥ 2) or unrelated advanced/severe medical comorbidities (such that overall life expectancy is limited and thermal ablation would not confer significant survival benefit), uncorrectable coagulopathy, absolute contraindications to intravenous (IV) contrast (limiting evaluation of treatment margins during or after treatment), or an inability to tolerate moderate sedation or general anesthesia. Technical contraindications include an inability to ablate the entire lesion, either due to location or to the risk of nontarget thermal ablation that cannot be addressed by adjunctive techniques (to be described). For example, proximity to central biliary ducts (within 1 cm) can place patients at higher risk for developing main right or left hepatic duct strictures. 2 Patients with more extensive disease (larger tumors, > 5 tumors in number) should be evaluated for other therapies (e.g., transarterial yttrium-90 radioembolization). Finally, there is currently no evidence that use of ablation for large tumor debulking has any benefit in overall survival.
7.4 Patient Selection and Preprocedure Evaluation
Multiple potential treatment options are available for patients with hepatic colorectal metastases, often necessitating the need for determining optimal order and combination of treatment (e.g., preoperative or preablation chemotherapy, combination ablation and surgical resection). As such, a prospective multidisciplinary review of patients with metastatic colorectal cancer is strongly recommended and should involve medical oncologists, colorectal and hepatobiliary surgeons, and interventional radiologists. This will help in planning the best patient-specific treatment course, and it also allows the interventional radiologist to present ablation as a treatment option early in the disease course (where it is most beneficial).
7.4.1 Initial Clinical Evaluation
All patients should undergo careful evaluation prior to any procedure, including a thorough history and physical examination. 3 This should include a formal initial outpatient clinic visit that allows the physician to carefully evaluate the patient for procedure suitability, plan the procedure itself, discuss ablation in detail with the patient (including a thorough discussion of likelihood of outcome and potential complications), obtain informed consent, and map out the course for postprocedure care and follow-up imaging.
During an initial clinical evaluation, a detailed history and physical examination should be performed. 3 First, a detailed oncological history should be obtained, including initial presentation, symptoms related to the tumor and/or treatment, current staging and extent of the tumor involvement, and treatment to date (e.g., systemic chemotherapy regimens and number of cycles administered, primary tumor resection, or hepatic resection). Next, an assessment of overall performance status should be performed. ECOG and Karnofsky scoring systems are commonly used oncological tools to grade the impact of the patient’s disease and overall functional status. 4 , 5 Overall, a falling performance status score is indicative of a poor prognosis and should be considered when an ablative procedure is planned in the context of the overall goals of care. Third, a clinical review should focus on issues that will affect if and how ablation can be performed. This includes a review of medical comorbidities that will influence the level of procedure complexity (such as prior hepatic or biliary surgery), choice of moderate sedation or general anesthesia use, patient positioning (e.g., prior back or joint surgeries), and use of contrast (medical diseases that predispose to chronic renal insufficiency, such as diabetes and hypertension). Medications and allergies should also be reviewed, specifically screening for use of anticoagulation (e.g., antiplatelet agents, Coumadin [Bristol-Myers Squibb Company], and heparin), medications that need to be stopped periprocedurally (e.g., metformin), and prior reactions to contrast.
Preprocedure laboratory testing should be obtained in all patients, including a complete blood count (CBC), serum chemistry profile, renal function tests, coagulation tests, and liver function tests. Some degree of hepatic dysfunction can be common in patients treated with systemic chemotherapy. Chemotherapy-associated steatohepatitis (CASH) can occur in up to 20% of patients who undergo treatment with regimens that include oxaliplatin and irinotecan. 6 Aberrant coagulation needs to be identified and corrected appropriately prior to the procedure to minimize bleeding complications. Carcinoembryonic antigen (CEA) levels are elevated in patients with colorectal cancer–related hepatic metastases, and baseline levels should be established prior to ablation because this can identify patients who have responded to initial chemotherapy, stratify those in whom early use of ablation may yield better outcomes, 7 and make it possible to follow trends after treatment and to detect development of new metastases. 8
Finally, all available imaging should be reviewed to determine the overall number of tumors and exclude evidence of extrahepatic disease. Prior to the procedure, all patients should have undergone recent (within the last 2 weeks) contrast-enhanced cross-sectional computed tomographic (CT) or magnetic resonance imaging (MRI) scans. Knowing the original extent of intrahepatic disease (including tumor size and number), some of which may be smaller or not visible on most recent imaging studies, is important in planning the extent of ablation required. Additionally, imaging should be reviewed to plan probe placement and identify factors (central location, subcapsular tumor, nearby organs such as the gallbladder or colon) that might make tumor targeting more difficult.
7.4.2 Neoadjuvant/Adjuvant Chemotherapy
Neoadjuvant chemotherapy can be given in patients who are not suitable candidates for ablation on initial assessment, with interval follow-up to assess for potential downstaging of their tumor burden for resection/ablation. One study has reported a 5-year survival of 34% with use of neoadjuvant chemotherapy followed by radiofrequency ablation (RFA). 9 However, ablation of residual tumor may be difficult given tumor shrinkage and reduced visibility in the setting of global CASH-related changes, and larger ablative margins are often required to treat nonvisible microscopic residual tumor at the margins. Ablating tumors that have completely disappeared with chemotherapy can be very challenging, and close surveillance may be required with plans to treat tumors that reemerge at those sites. 10 Based on studies suggesting some benefit for adjuvant chemotherapy surrounding hepatic resection, adjuvant chemotherapy postablation has also been reported and likely confers some benefit. Studies have reported median survival times of up to 48 months after RFA followed by adjuvant chemotherapy for unresectable colorectal liver metastases. Others have similarly reported improvement in overall survival with RFA followed by adjuvant chemotherapy of 9 months (28 vs. 19 months after laparoscopic ablation), 11 and 10-year survival of 18% using RFA with adjuvant 5-fluorouracil (5-FU)/irinotecan. 1 Given low morbidity associated with percutaneous ablation, adjuvant chemotherapy can be started shortly after (within 2 weeks), or if patients are on chemotherapy, it can be continued through the periablation period. In summary, ablation with chemotherapy is generally preferred over either alone (if tolerated).
7.4.3 Choice of Sedation
Hepatic ablation procedures can be performed under conscious sedation or general anesthesia. In general, uncomplicated procedures can be performed under conscious sedation. However, thermal ablation (particularly heat-based modalities using radiofrequency and microwave) within the liver can be quite uncomfortable and may not be adequately managed with moderate sedation in some cases. Use of general anesthesia should be considered in (1) patients with significant medical comorbidities, corresponding to an American Society of Anesthesiologists (ASA) physical status score of IV 3 ; (2) patients who have a high tolerance for pain or are routinely taking narcotic pain medications; (3) procedures that are likely to cause procedural pain that will not be well controlled with moderate sedation, as for subcapsular tumors or tumors adjacent to the diaphragm; (4) patients with a difficult airway, placing them at risk for airway compromise with moderate sedation, as denoted by a Mallampati airway classification of IV 12 ; (5) patients with specific comorbidities that require careful airway and ventilator management during sedation (e.g., morbid obesity, large pleural effusions, or sleep apnea); and (6) ablations with a predicted long procedure time or complicated technical elements (e.g., requiring multiple electrodes, repositioning, and/or overlapping ablations). 13 The procedure plan should be discussed beforehand with the anesthesiologist so that the choice of anesthesia is matched to the needs of the procedure. This should include discussing the use of monitored anesthesia care (MAC) or general anesthesia with endotracheal intubation, the use of paralytics (necessary for breath holds), patient positioning, and the use of regional paravertebral blocks. 3 , 14 , 15
7.4.4 Preprocedure Antibiotics
Although the benefit of preprocedural antibiotics remains unclear, administering single-dose antibiotics immediately before the procedure is likely indicated. 16 A specific subset of patients are at higher risk, including those who have had prior hepatic abscesses or diabetes mellitus or who have undergone surgical biliary reconstructive or biliary-enteric bypass procedures (e.g., hepaticojejunostomies). 3 Different pre- and postablation antibiotic regimens for preventing hepatic abscess in patients with biliary-enteric anastomoses have been reported with mixed success. At our institution, we use one of two regimens that have had reported success in small series for transarterial chemoembolization (a population with similarly increased risk of posttreatment abscess) either using (1) a combination of bowel preparation and IV antibiotics (IV piperacillin/tazobactam or cefotetan with IV metronidazole) 17 or (2) oral moxifloxacin (an agent with broad-spectrum gram-negative coverage and biliary excretion) given 3 days before to 21 days after treatment. 18
7.5 Hepatic Ablation Technique
Performing successful hepatic ablations requires being familiar with what the overall goals of ablation therapy are (including definitions of a successful treatment), available ablation modalities and energy sources, common methods of intraprocedural imaging guidance, planning probe placement, and immediate postprocedure imaging evaluation.
7.5.1 Key Objectives of Hepatic Ablation
First, the main goal of hepatic ablation is inducing focal cytotoxic injury (by placing needle-like applicators at the center of the tumor to generate high temperatures [> 60°C]) throughout the entire target tumor. 19 An appropriate “ablative” margin is necessary to adequately treat a rim of apparently “normal” tissue around the tumor, which often contains microscopic invasion of malignant cells at the tumor periphery. For hepatic colorectal metastases, this rim should be 10 mm thick extending circumferentially beyond the edge of visible tumor. 20 This means that for 3 to 5 cm tumors, a single ablation treatment may not be sufficient to entirely encompass the target volume. 21 In these cases, multiple overlapping ablations or simultaneous use of multiple applicators may be required to successfully treat the entire tumor and achieve an ablative margin. 22 Additionally, if metastases have decreased in size from chemotherapy before ablation is being performed, the ablation zone should be sufficiently large to include the area originally involved by the tumor. The second objective is to ensure accuracy and minimize damage to nontarget normal tissue, an advantage over surgical resection. This is particularly important in patients who have undergone extensive hepatic resections for liver metastases and then develop new tumors in the remaining liver remnant. Third, tumor ablation should be performed to treat the entire target while simultaneously minimizing the potential for nontarget thermal injury to nearby structures both inside and outside the liver. Careful planning is required to ensure that each of these three objectives is met.
7.5.2 Selection of Ablation Modality
A detailed discussion of different types of ablation modalities is beyond the scope of this chapter. However, it is important to be familiar with two types of ablation platforms that are most commonly used for hepatic ablation of colorectal metastases—radiofrequency (RF) or microwave (MW) energies. Both types of ablation elevate tissue temperatures sufficient to create zones of irreversible cellular damage. RF energy is easy to generate, but it suffers in areas of high blood flow or high tissue impedance and requires use of electrode switching or multiple-applicator use to achieve large ablation zones in a time-efficient manner. MW heating is fast and efficient and thus appears better equipped to overcome heat sinks and treat large tumor volumes. In current clinical practice, RF-based platforms are most commonly used and have the largest set of relevant long-term clinical data (i.e., studies were performed with systems still used in clinical practice). However, there are now a number of commercially available MW-based systems, with data increasingly demonstrating at least equivalency to RFA, and potentially superiority for larger tumors. This is particularly relevant in treating colorectal liver metastases, where the need to achieve a 10 mm ablative margin necessitates larger ablation zones (e.g., 5 cm ablations for 3 cm tumors). Finally, operator technique likely has as much influence on performance as device technology; for example, multiple applicators can be used to increase heating rather than switching to a different energy source.
7.5.3 Intraprocedural Image Guidance and Monitoring
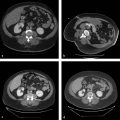
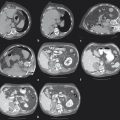
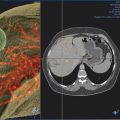
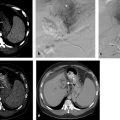
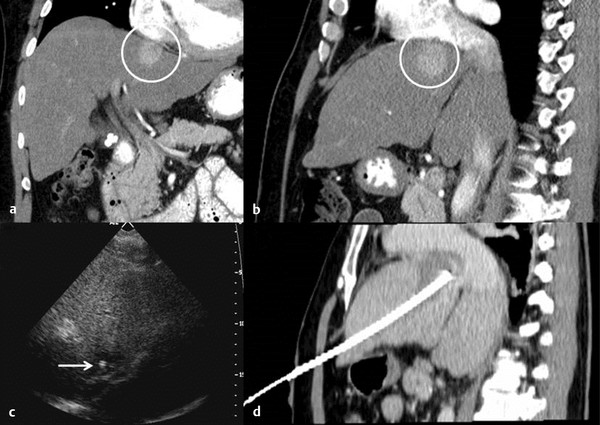
Hepatic ablation procedures are commonly performed under ultrasound (US), CT (with or without fluoroscopy), or combination US and CT guidance (preferred in our practice) ( Fig. 7.1 ). Selection of the appropriate imaging modality depends on optimal tumor visibility. Real-time US guidance is very useful in electrode placement and positioning during the procedure but may provide limited visibility of tumors in certain locations, such as the hepatic dome. Additionally, US provides limited information on monitoring success of the procedure as the area of treatment becomes obscured by echogenic foci of gas generated by the ablation. CT with or without fluoroscopy can be helpful in targeting deep tumors or in cases where a difficult access trajectory is required. However, target tumors are often difficult to visualize on noncontrast CT images. IV contrast can also be administered at the time of the procedure to confirm target location.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree