4 Hepatocellular Carcinoma: Ablation
4.1 Introduction
Hepatocellular carcinoma (HCC) is currently the second leading cause of cancer-related death worldwide. 1 Unlike most solid cancers, future incidence and mortality rates for HCC were projected to largely increase in several regions around the world over the next decade. 2 , 3
HCC is increasingly diagnosed at an early, asymptomatic stage owing to surveillance of high-risk patients. 4 Patients with early-stage HCC require careful therapeutic management. Given the complexity of the disease, multidisciplinary assessment of tumor stage, liver function, and physical status is required for proper therapeutic planning. Patients with early-stage HCC should be considered for any of the available curative therapies, including surgical resection, liver transplantation, and image-guided ablation. 5 , 6 , 7
Image-guided ablation is recommended for patients with early-stage HCC when surgical options are precluded. 5 , 6 , 7 Although tumor ablation procedures can be performed at laparoscopy or surgery, most procedures aimed at treating HCC are performed with a percutaneous approach. Hence several authors refer to these procedures as percutaneous therapies. Over the past 25 years, several methods for focal tumor destruction have been developed and clinically tested. 8 Although radiofrequency ablation (RFA) has been the most popular technique, several alternate technologies, including thermal and nonthermal methods, have recently attracted attention because they appear to be able to overcome some specific limitations of RFA. 9 , 10 , 11
4.2 Indications and Contraindications
A multidisciplinary team must perform a careful clinical, laboratory, and imaging assessment of each individual patient to evaluate the patient’s eligibility for image-guided ablation. Laboratory tests should include a full evaluation of the patient’s coagulation status. A prothrombin time ratio > 50% and a platelet count > 50,000/µL are required to keep the risk of bleeding at an acceptably low level. The tumor staging protocol should include triple-phase computed tomography (CT) or magnetic resonance imaging (MRI) of the liver as well as imaging examinations to rule out extrahepatic disease, as appropriate.
To be considered for image-guided ablation, patients should have either a single tumor smaller than 5 cm or as many as three nodules smaller than 3 cm each, no evidence of vascular invasion or extrahepatic spread, an Eastern Cooperative Oncology Group (ECOG) performance status test of 0 or 1, and liver cirrhosis in Child–Pugh class A or B. 5 , 6 , 7 Pretreatment imaging must carefully define the location of each lesion with respect to surrounding structures. Lesions located along the surface of the liver can be considered for thermal ablation, although their treatment requires adequate expertise and may be associated with a higher risk of complications. Thermal ablation of superficial lesions that are adjacent to any part of the gastrointestinal tract must be avoided because of the risk of thermal injury of the gastric or bowel wall. The colon appears to be at greater risk than the stomach or small bowel for thermally mediated perforation. Gastric complications are rare, likely owing to the relatively greater wall thickness of the stomach or the rarity of surgical adhesions along the gastrohepatic ligament. Thermal ablation of lesions adjacent to the gallbladder or to the hepatic hilum is at risk of thermal injury of the biliary tract. In experienced hands, thermal ablation of tumors located in the vicinity of the gallbladder was shown to be feasible, although associated in most cases with self-limited iatrogenic cholecystitis. In contrast, thermal ablation of lesions adjacent to hepatic vessels is possible because flowing blood usually protects the vascular wall from thermal injury. In these cases, however, there may be an increased risk of incomplete treatment of the neoplastic tissue close to the vessel because of the heat loss by convection.
Further studies are needed to establish whether novel technologies will expand the clinical role of image-guided ablation with respect to thermal ablation—RFA and microwave ablation (MWA). In particular, promising data have been reported on the safety and efficacy of nonthermal technologies, such as irreversible electroporation (IRE), in treating tumors in proximity to vital structures. It has to be pointed out, however, that the oldest and cheapest ablation technique, percutaneous ethanol injection (PEI), remains a viable option for small HCC tumors unsuitable for thermal ablation.
4.3 Technique
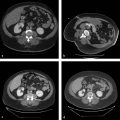
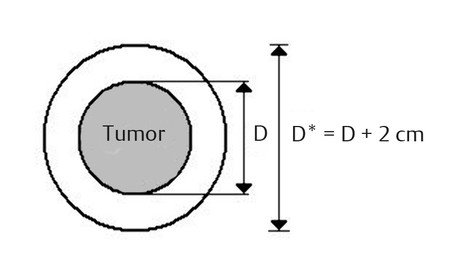
RFA is currently established as the standard ablative modality. 12 , 13 However, several alternate energy-based methods for focal tissue destruction have recently become available. These novel ablation technologies destroy a tumor either through thermal (heat or cold) or nonthermal mechanisms. Whatever the ablative modality, the ablation of appropriate margins beyond the visible borders of the tumor is necessary to achieve therapeutic results similar to those achieved with surgery. Ideally, a 360°, 0.5 to 1 cm thick ablative margin should be produced all around the target tumor. This cuff would ensure that the peripheral portion of the lesion as well as any microscopic invasions located in its close proximity have been eradicated ( Fig. 4.1 ).
RFA systems function in the 375 to 500 KHz range. Most devices currently used are monopolar in that there is a single active electrode, with current dissipated at one or more return grounding pads. Bipolar devices have two active electrode applicators, usually placed in close proximity to achieve contiguous coagulation between the two electrodes, or on a single electrode. Several electrode types are available for clinical RFA, including internally cooled electrodes and multitined expandable electrodes with or without perfusion. 12 , 13
MWA systems function at 915 MHz or 2.45 GHz frequencies. These wavelengths have specific physical properties with regard to tissue permeability and antenna design. Microwaves can generate very high temperatures in very short time periods, potentially leading to improved treatment efficiency and larger ablation zones, with less susceptibility to heat sink effects. Multiple microwave antennas can be powered simultaneously to take advantage of thermal synergy when placed in close proximity or widely spaced to ablate several tumors simultaneously.
The term cryoablation should be exclusively used for all methods of destroying tissue by the application of low temperature freezing, or alternating freezing and thawing or slight heating. Rapid tissue freezing and thawing produce the greatest cytotoxic effects by disrupting cellular membranes and inducing cell death. A distinct advantage of cryoablation is the ability to monitor the ice-ball formation during the procedure.
IRE includes those technologies and devices that cause cell death through the repeated application of short-duration high-voltage electrical pulses that create irreversible injuries to cellular membranes. Although there may be some hyperthermic ablative changes with higher power applications, the mechanism of cell death with IRE is thought to be predominantly nonthermal. Hence, issues associated with perfusion-mediated tissue cooling are not relevant for this technology. IRE is administered under general anesthesia with administration of a neuromuscular blocking agent to prevent undesirable muscle contraction, and by using cardiac gating to synchronize pulse delivery with the absolute refractory period to prevent cardiac arrhythmias.
Other energy-based ablation technologies include laser ablation and high-intensity focused ultrasound. These technologies have been adopted by a few centers worldwide for the treatment of malignant liver tumors.
4.4 Imaging
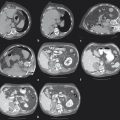
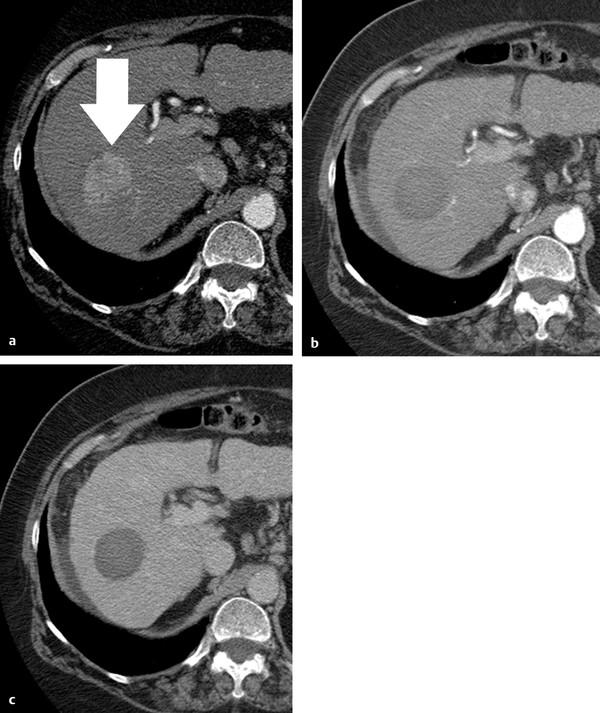
Targeting of the lesion can be performed with ultrasound, CT, or MRI ( Fig. 4.2 ). The guidance system is chosen largely on the basis of operator preference and local availability of dedicated equipment, such as CT fluoroscopy or open MRI systems. Real-time ultrasound/CT (or ultrasound/MRI) fusion imaging systems may improve the ability to guide and monitor liver tumor ablation procedures. During the procedure, important aspects to be monitored include how well the tumor is being covered and whether any adjacent normal structures are being affected at the same time. Although the transient hyperechoic zone that is seen at ultrasound within and surrounding a tumor during and immediately after RFA can be used as a rough guide to the extent of tumor destruction, MRI is currently the only imaging modality with validated techniques for real-time temperature monitoring.

Contrast-enhanced imaging performed at the end of the procedure may allow an initial evaluation of treatment effects. However, 1-month CT or MRI examinations are recognized as the standard to assess treatment outcome. CT and MRI show successful ablation as a nonenhancing area with or without a peripheral enhancing rim ( Fig. 4.3 ). The enhancing rim that may be observed along the periphery of the ablation zone appears as a relatively concentric, symmetric, and uniform process in an area with smooth inner margins. This is a transient finding that represents a benign physiological response to thermal injury (initially, reactive hyperemia; subsequently, fibrosis and giant cell reaction). Benign periablational enhancement needs to be differentiated from irregular peripheral enhancement due to residual tumor that occurs at the treatment margin. In contrast to benign periablational enhancement, residual unablated tumor often grows in scattered, nodular, or eccentric patterns. Later follow-up imaging studies should be aimed at detecting the recurrence of the treated lesion (i.e., local tumor progression), the development of new hepatic lesions, or the emergence of extrahepatic disease.
4.5 Complications
Several multicenter surveys have reported acceptable morbidity and mortality rates for RFA of liver tumors. The mortality rate ranged from 0.1 to 0.5%, the major complication rate ranged from 2.2 to 3.1%, and the minor complication rate ranged from 5 to 8.9%. 14 The most common causes of death were sepsis, hepatic failure, colon perforation, and portal vein thrombosis, while the most common complications were intraperitoneal bleeding, hepatic abscess, bile duct injury, hepatic decompensation, and grounding pad burns. 15 , 16 , 17 Minor complications and side effects were usually transient and self-limiting. An uncommon late complication of RF ablation can be tumor seeding along the needle track. In patients with HCC, tumor seeding occurred in 8 (0.5%) of 1,610 cases in a multicenter survey. 15 Lesions with subcapsular location and an invasive tumoral pattern, as shown by a poor differentiation degree, seem to be at higher risk for such a complication. 18 Although these data indicate that RFA is a relatively safe procedure, a careful assessment of the risks and benefits associated with the treatment has to be made in each individual patient by a multidisciplinary team.
4.6 Clinical Data and Outcomes
The use of RFA for local ablation of early-stage HCC is supported by a large amount of data and robust clinical evidence. Five randomized, controlled trials (RCTs) have compared RFA versus PEI for the treatment of early-stage HCC. These investigations consistently showed that RFA has a higher anticancer effect than PEI, leading to a better local control of the disease 19 , 20 , 21 , 22 , 23 ( Table 4.1 ). The assessment of the impact of RFA on survival has been more controversial. Although a survival benefit was identified in the three RCTs performed in Asia, the two European RCTs failed to show statistically significant differences in overall survival between patients who received RFA and those treated with PEI, despite the trend favoring RFA ( Table 4.1 ). In patients with early-stage HCC treated with percutaneous ablation, long-term survival is often the result of different sequential interventions, given that about than 80% of the patients will develop recurrent intrahepatic HCC nodules within 5 years of the initial treatment and will received additional therapies. 24 Nevertheless, three independent meta-analyses including all RCTs have confirmed that treatment with RFA offers a survival benefit as compared with PEI, particularly for tumors larger than 2 cm, thus establishing RFA as the standard percutaneous technique. 25 , 26 , 27 For studies that reported major complications, however, the incidence in RFA-treated patients was 4.1% (95% confidence interval [CI], 1.8–6.4%), compared to 2.7% (95% CI, 0.4–5.1%) observed in PEI-treated patients. 28 This difference was not statistically significant; nevertheless, this safety profile should be taken into consideration as part of the overall risk/benefit profile in each case.
Reference | Initial CR | Treatment failurea | Overall survival | ||
1 y | 3 y | P | |||
Lencioni et al 19 | |||||
RFA (n = 52) | 91% | 8% | 88 | 81 | NS |
PEI (n = 50) | 82% | 34% | 96 | 73 | |
Lin et al 20 | |||||
RFA (n = 52) | 96% | 17% | 82 | 74 | 0.014 |
PEI (n =52) | 88% | 45% | 61 | 50 | |
Shiina et al 21 | |||||
RFA (n = 118) | 100% | 2% | 90 | 80 | 0.02 |
PEI (n = 114) | 100% | 11% | 82 | 63 | |
Lin et al 22 | |||||
RFA (n = 62) | 97% | 16% | 88 | 74 | 0.031 |
PEI (n = 62) | 89% | 42% | 96 | 51 | |
Brunello et al 23 | |||||
RFA (n = 70) | 96% | 34% | 88 | 59 | NS |
PEI (n = 69) | 66% | 64% | 96 | 57 | |
Abbreviations: CR, complete response; NS, not significant; PEI, percutaneous ethanol injection; RFA, radiofrequency ablation. aIncludes initial treatment failure (incomplete response) and late treatment failure (local recurrence). | |||||
Recent reports on long-term outcomes of RFA-treated patients have shown that, in patients with Child–Pugh class A and early-stage HCC, 5-year survival rates are as high as 51 to 64% and may reach 76% in patients who meet the Barcelona Clinic Liver Cancer (BCLC) criteria for surgical resection 24 , 29 , 30 , 31 ( Table 4.2 ). Caution, however, is needed when interpreting and generalizing these results, in particular in light of studies that suggest a nonnegligible rate of incomplete histopathological response after RFA. In fact, the ability of RFA to achieve complete tumor eradication appears to be dependent on tumor size and location. In particular, histological studies performed in liver specimens of patients who underwent RFA as a bridge treatment to transplantation showed that the presence of large (≥ 3 mm) abutting vessels result in a drop of the rate of complete tumor necrosis to < 50% because of the heat loss due to perfusion-mediated tissue cooling within the area to be ablated. 32
Patients | Overall survival | |||
Reference | ||||
no. | 1 year | 3 years | 5 years | |
Lencioni et al 24 | ||||
Child-Pugh A | 144 | 100 | 76 | 51 |
Child-Pugh B | 43 | 89 | 46 | 31 |
Tateishi et al 29 | ||||
Child-Pugh A | 221 | 96 | 83 | 63 |
Child-Pugh B-Ca | 98 | 90 | 65 | 31 |
Choi et al 30 | ||||
Child–Pugh A | 359 | NA | 78 | 64 |
Child–Pugh B | 160 | NA | 49 | 38 |
N’Kontchou et al 31 | ||||
BCLC resectableb | 67 | NA | 82 | 76 |
BCLC unresectable | 168 | NA | 49 | 27 |
Abbreviation: BCLC, Barcelona Clinic Liver Cancer. aOnly 4 of 98 patients had Child-Pugh C cirrhosis. bBCLC criteria for resection include single tumor, normal bilirubin level (< 1.5 mg/dL), and absence of significant portal hypertension. | ||||
Several attempts have been made to increase the effect of RFA in HCC treatment. Because heat efficiency is the difference between the amount of heat produced and the amount of heat lost, most investigators devote their attention to strategies that aim primarily at minimizing heat loss due to perfusion-mediated tissue cooling. Given that HCC is mostly nourished by the hepatic artery, a combination of RFA and balloon catheter occlusion of the tumor arterial supply or prior transarterial chemoembolization (TACE) has been used to increase heat efficiency. 33 , 34 , 35 , 36 The combination of TACE and RFA did not show significant clinical benefit when applied to the treatment of small (< 3 cm) HCC. 37 However, such an approach did show significant advantages, in terms of both local tumor control and survival, when used in intermediate-sized (3–5 cm) HCC 38 , 39 as well as in recurrent HCC after prior hepatectomy. 40
The largest RCT conducted so far included 189 patients with HCC < 7 cm. 41 Patients were randomly assigned to receive TACE combined with RFA (TACE-RFA; n = 94) or RFA alone (n = 95). The 1-, 3-, and 4-year overall survivals for the TACE-RFA group and the RFA group were 92.6%, 66.6%, and 61.8% and 85.3%, 59%, and 45.0%, respectively. The corresponding recurrence-free survivals were 79.4%, 60.6%, and 54.8% and 66.7%, 44.2%, and 38.9%, respectively. Patients in the TACE-RFA group had better overall survival and recurrence-free survival than patients in the RFA group (hazard ratio [HR], 0.525; 95% CI, 0.335–0.822; P = 0.002; HR, 0.575; 95% CI, 0.374–0.897; P = 0.009, respectively). On logistic regression analyses, treatment allocation, tumor size, and tumor number were significant prognostic factors for overall survival, whereas treatment allocation and tumor number were significant prognostic factors for recurrence-free survival.
Although TACE followed by RFA has been the most popular combination, alternate strategies have been investigated. In particular, drug-eluting bead (DEB)-TACE has been performed after RFA, rather than before, following a different rationale. 42 In a standard RFA, one can take advantage of only those temperatures that are sufficient by themselves to induce coagulative necrosis (> 50–55°C). However, there are large zones of sublethal heating created during RF application in tissues surrounding the electrode, that are not being used to achieve sustained treatment effect. Experimental studies in animal tumor models have shown that lowering the temperature threshold at which cell death occurs by combining sublethal temperature with cell exposure to chemotherapeutic agents increases tumor necrosis, apparently occurring in tissues heated to 45 to 50°C. It has been hypothesized that the administration of DEB to tumors incompletely killed by RFA could increase the anticancer effect, as a result of both the delivery of highly concentrated doxorubicin into a relatively small volume of residual viable neoplastic tissue and the reduced cell resistance to the drug caused by the exposure to sublethal heating.
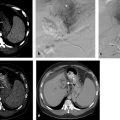
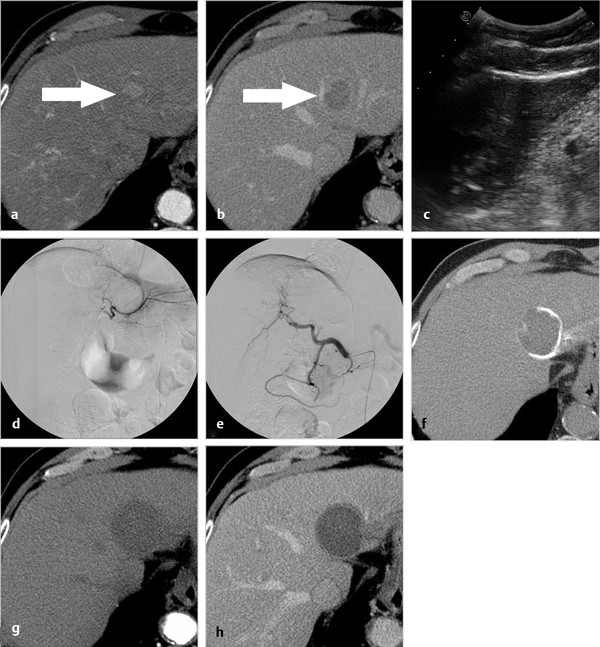
In a pilot clinical study, DEB-TACE was performed within 24 hours of RFA to take advantage of the reactive hyperemia induced by RF application to facilitate delivery of the microspheres to the tumor-bearing area. In fact, marked periablational hypervascularity was observed at the time of the angiographic study. DEB administration resulted in a substantial increase in tissue destruction, leading to confirmed complete response (CR) of the target lesion in 12 (60%) of 20 patients bearing large tumors refractory to standard RF treatment 42 ( Fig. 4.4 ). DEB-enhanced RFA was well tolerated, with no major complications associated with the technique. Nevertheless, after this initial evaluation of efficacy and safety, further clinical investigation is warranted to prove the clinical benefit of this approach.
Is RFA still the best technique for tumor ablation? Several novel alternatives are currently being tested clinically. MWA, in particular, is emerging as a valuable alternative to RFA and seems to have potential to improve the rate of complete ablation achieved with RFA in multiple tumors or in tumors that are > 2 to 3 cm as well as tumors in a perivascular location. 43 , 44 A review of the literature on the use of MWA for hepatic tumors included published studies in the English language with at least 30 patients per study. 43 The authors concluded that MWA may be optimal when larger necrosis zones and/or ablation of multiple lesions are the objective. The data reviewed supported the potential advantage noted for ablation of HCC lesions > 3 cm, but did not support treatment of HCC lesions < 3 cm. 43 In a recent multi-institutional study including 450 patients treated with 473 MWA procedures in four leading U.S. institutions (139 HCC, 198 colorectal cancer (CRC) liver metastases, 61 neuroendocrine liver metastases, and 75 other), complete ablation was confirmed for 839 of 865 tumors (97%) on follow-up cross-sectional imaging, and the local recurrence rate was 6%. 44 In another large series including 100 patients, a matched comparison of MWA and RFA was performed. 45 The two groups were evaluated for ablation success, ablation recurrence, and ablation time, all of which were significantly improved in the MWA group. Ablation and operative times were significantly shortened in the MWA group, primarily related to the ability to do simultaneous ablations with multiple probes. The authors concluded that MWA of hepatic tumors is a safe and effective method for treating unresectable hepatic tumors, with a low rate of local recurrence.
IRE shows promise for the treatment of small tumors located in the vicinity of bile ducts and blood vessels. 46 , 47 , 48 In the first clinical study published on the use of IRE in liver tumor treatment, factors analyzed included patient and tumor characteristics, treatment-related complications, and local recurrence-free survival for ablated lesions. The series included 44 patients. 46 Tumor histologies included CRC metastasis (n = 20), HCC (n = 14), and others (n =10). Initial success was achieved in 46 (100%) treatments. Five patients had nine adverse events, with all complications resolving within 30 days. There was a trend toward higher recurrence rates for tumors > 4 cm (HR 3.236, 95% CI: 0.585–17.891; P = 0.178). A recent systematic review of safety and efficacy of IRE concluded that, in cases where other techniques are unsuitable, IRE is a promising modality for the ablation of tumors near bile ducts and blood vessels. 48
More data are needed to define the potential for other energy-based ablation technologies, such as cryoablation, in the specific field of liver tumor treatment. A recent safety study on 42 patients with HCC reported 13 (39.4%) complications out of 33 cryoablation procedures versus 8 (26.7%) complications out of 30 RFA procedures, including 2 (6.1%) severe/fatal complications for cryoablation procedures versus 1 (3.3%) for RFA. The authors concluded that no significant difference was seen in the overall safety of cryoablation and RFA in the treatment of HCC in patients with cirrhosis. 49
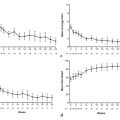
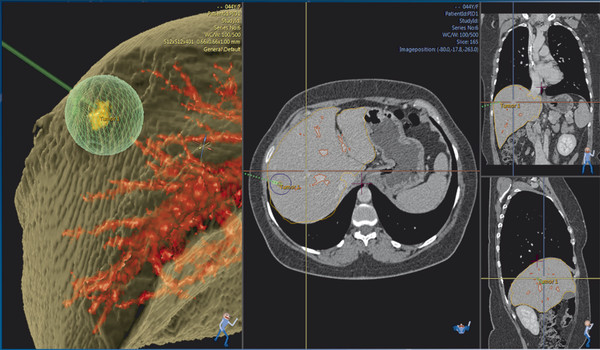
Advances in ablation systems and devices are highly warranted. However, progress in imaging guidance and monitoring is also key to success. To be able to compete with surgical resection, image-guided ablation needs to be able to offer more accurate prediction of the outcome in each individual patient. Variability in outcomes needs to be minimized via careful treatment planning ( Fig. 4.5 ). Also, the outcome of the ablation procedure needs to be carefully documented by providing sound evidence that an “A0” treatment has been achieved.
Pearls
Indication to use image-guided ablation should be discussed by a multidisciplinary team after careful assessment of tumor stage, liver function, and performance status.
Accurate pretreatment planning, including selection of the ablation technology, imaging guidance, and approach, is key to success.
Ablation protocols are usually based on the ablation size charts provided by the manufacturer. However, the actual ablation zone may vary in clinical setting, depending on tissue vascularization, tissue conductivity, local interactions, and settings of the system, among other factors.
Tumors are generally assumed to be spherical. If the difference between the longest axis and the shortest axis of a tumor is 1 cm or greater, appropriate changes to the ablation strategy may be warranted in view of the ellipsoidal shape of the target.
Imaging aspects that are particularly important include tumor size, shape, and location within the organ relative to blood vessels as well as to critical structures that might be at risk for injury.
Changes in the actual position of the electrodes with respect to planned position can potentially lead to incomplete ablation or thermal injury of structures located in the vicinity of the target tumor: appropriate changes to the ablation protocol may be warranted based on the actual position of the electrodes.
Posttreatment response assessment and follow-up protocols are fundamental components of the treatment strategy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree