3 Ablation of Lung Cancer, Pulmonary Metastatic Disease, and Chest Wall Malignancy
3.1 Introduction
The role of thermal ablation in the treatment of thoracic malignancies has been growing for more than a decade. It has become an established treatment alternative to surgical resection in patients with both primary lung cancer and metastatic disease, as well as in patients with locally recurrent tumor following treatment. Emerging indications include treatment of primary and secondary tumors of the pleura and chest wall, as well as palliative ablation for painful soft tissue and bone lesions.
For early-stage, non–small cell lung cancer (NSCLC) (stage I/II), surgical resection or lobectomy remains the treatment of choice. 1 However, many patients are deemed medically inoperable for lobectomy or refuse surgery. Studies have shown that patients who receive some form of therapy for early-stage lung cancer demonstrate better outcomes than those who do not receive any form of therapy. 2 Therefore, a sizeable proportion of medically inoperable, early-stage lung cancer can benefit from less invasive therapies, such as percutaneous thermal ablation.
Approximately 20% of patients with lung metastases and resected primary soft tissue tumors have a limited number of metastases isolated to the lung and are potential candidates for surgical resection or metastasectomy. 3 Although surgical metastasectomy may result in improved disease-free survival in properly selected patients, multiple surgical resections may diminish pulmonary capacity. 4 Therefore, lung-sparing techniques, including percutaneous thermal ablation, are an attractive option in those candidates with limited metastatic disease or limited recurrent or residual disease following prior surgical resection, chemotherapy, or radiotherapy.
Management of malignancies of the chest wall and pleura is a promising new and not fully evaluated indication for thoracic tumor ablation. A growing body of data supports the efficacy of thermal ablation in treating soft tissue lesions, 5 and application to these tissues in the thorax is a natural extension. Although the published data specifically addressing the application of thermal ablation to pleural and chest wall disease are limited, our institutional experience has yielded promising results in the treatment of thymoma, mesothelioma, and metastatic disease, including, but not limited to, chondrosarcoma.
3.2 Principles of Thermal Ablation
3.2.1 Radiofrequency Ablation
Radiofrequency ablation (RFA) is based on a thermal energy delivery system that causes agitation of ionic dipolar molecules, resulting in frictional heating of the surrounding tissues and fluids. The primary challenge in the application of RF energy to tumors in the lung is the disparity of tissue characteristics between normally aerated lung and tumor. The lung acts as an insulator, due to its naturally high impedance, which can limit the extension of an ablation zone to include a margin, compromising the likelihood of local treatment success. This insulation by the normal lung has the positive effect of increasing the central temperature of a solid mass that has been directly punctured by the ablation needle, the so-called oven-effect. 6
Pleural and chest wall tumors tend to have characteristics similar to those of adjacent tissue; therefore the ablation zone tends to be more predictable. The potential traversal of nerves and the possibility of nerve injury (e.g., phrenic nerve, brachial plexus), related either to electrode placement or to ablation, are important considerations in treatment planning and delivery. 7 On a practical level, heat-based ablations, particularly RFA, can result in significant intraprocedural discomfort, which may require increased conscious sedation, and, possibly, general anesthesia. Additionally, some patients may experience postablative pain secondary to tissue charring, and, occasionally, neuropathic pain from nerve ablation. 8 , 9
3.2.2 Microwave Ablation
Microwave ablation (MWA) is performed by microwave energy (900–2,450 MHz) inducing dipole excitation, which in turn causes the water molecules to spin, transferring some of their kinetic energy and creating friction, resulting in heat generation and tissue hyperthermia. 10 The microwave antenna emits electromagnetic radiation into tissue without the necessity of an electrical current; thus carbonization and gas pockets around the antenna do not interfere as much with energy deposition, resulting in higher intratumoral temperatures and more robust ablations as compared to RFA. 11
3.2.3 Percutaneous Cryotherapy
In contradistinction to the heat-based ablative techniques, percutaneous cryotherapy (PCT) uses dissipation of extreme cold temperatures as its mode of cell death. This results in a zone of cooling below freezing and ice ball formation, which is often visible on computed tomography (CT).
On a practical note, cryoablation systems allow for application of a “stick” mode, which decreases the probe temperature to − 10°C, forming a small ice ball and allowing for probe immobilization with respect to adjacent tumor or tissue. This can be used after satisfactory placement of a probe to facilitate additional probe placement, or to move the targeted tumor in lung parenchyma away from critical structures during cryoablation.
3.2.4 Special Equipment
Radiofrequency Ablation
Currently, commercially available RFA electrodes are either single-needle or multitined, with a gradual increasing trend toward use of single-needle electrodes. The zone of ablation may be increased for each probe by overlapping treatment regions for a single electrode, using programmed tine expansion, using multiple electrodes with a switch box generator, or diffusing hypertonic saline through specially designed electrodes into the tumor. Generally speaking, multiple electrodes should not be spaced farther than 2 cm apart in order to allow for complete coalescence of overlapping zones of ablation.
Dispersive, large surface area grounding pads are required and need to be carefully applied over the patient’s body, avoiding underlying bone prominences, and excess hair should be removed prior to pad placement to facilitate skin adhesion and ensure firm contact.
Microwave Ablation
Microwave generators allow single or multiple antennas to be activated simultaneously. Higher intratumoral temperatures are achieved as a result of an improved convection profile in comparison to RFA, resulting in larger ablation volumes with shorter ablation times with less heat sink phenomenon. No dispersive or grounding pads are required, and the absence of electrical stimulation, as with RFA, confers less pain as a result of nerve irritation, of particular importance when treating juxtapleural and chest wall lesions, which can often be quite painful during RFA as a result of intercostal nerve stimulation.
Percutaneous Cryoablation
There are two commercially available PCA systems that offer cryoprobes in sizes ranging from 1.2 to 3.8 mm in diameter. In theory, both systems achieve ice of similar dimension that is based on cryoprobe diameter; however, in our practice, the 2.4 mm probes are preferred to achieve adequate lethal ice.
PCA offers many advantages over the other thermal ablative techniques: the lack of electrical current allows for safe use in patients with implantable cardiac devices, the level of pain during and after ablation is decreased in comparison to RFA and MWA (of particular importance when addressing juxtapleural and pleural/chest wall tumors), and hypodense ice is easily visualized when ablating within soft tissues. Furthermore, some suggest that PCT is favored when addressing juxtapleural and paramediastinal lung lesions and pleural and chest wall lesions because it preserves tissue collagenous architecture. 12
An issue inherent to cryoablation is the relatively large-caliber probes (usually at least 2.4 mm or 13 G) compared to the 17 G heat-based probes, which can make skewering small tumors within the relatively more compliant lung difficult or impossible. This can be overcome by placement of at least two probes at the periphery of a lesion, the so-called chopstick approach, with commercially available generators able to accommodate up to 20 probes.
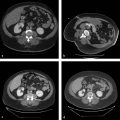
Within the lung, the authors use a triple-freeze technique (3-minute freeze–3-minute passive thaw–7-minute freeze–3-minute passive thaw–7-minute freeze) with intermittent short 3-minute passive thaw cycles after freeze cycles causing the airspaces to fill with pulmonary fluid and hemorrhage, significantly increasing thermal conductivity and allowing for extension of the ablation zone ( Fig. 3.1 ). In the chest wall and pleura, we typically employ a double-freeze technique with a longer, 8-minute passive thaw cycle, unless the anticipated ablative margin extends into the adjacent pulmonary parenchyma.
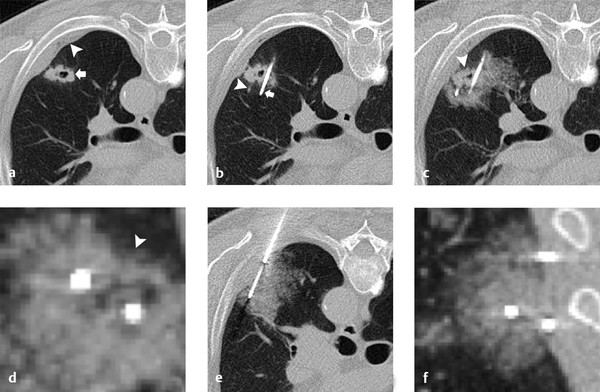
3.3 Indications
Proper patient selection is highly dependent on the anticipated goal to be achieved. Although no strict criteria for patient selection exists, emerging goals for percutaneous thermal ablation in the lung, chest wall, and pleura thus far have been established to include (1) potential for cure in early stage (stage I) NSCLCs that are medically inoperable or if the patient refuses surgery; (2) prolongation of survival in those with limited recurrent or metastatic disease to the lung and chest wall/pleura; (3) cytoreduction of large tumors to potentially alter the susceptibility of viable tumor tissue to chemotherapy or radiotherapy; (4) palliation of symptoms, particularly pain in peripheral tumors invading the pleural and chest wall 13 ; and (5) ablation of tumors adjacent to vital structures to limit or slow invasion.
3.4 Contraindications
Thoracic ablative procedures have few absolute and relative contraindications, which are similar to those of image-guided biopsy of the lung. Absolute contraindications include acute pneumonia, severe pulmonary arterial hypertension (> 40 mm Hg), and uncorrectable coagulopathy. Relative contraindications include poor lung function (forced expiratory volume in the first second of expiration [FEV1] < 1 L), prior pneumonectomy, or single-functioning lung, because the potential complications of pulmonary hemorrhage, pneumothorax, or hemothorax with poor or limited respiratory reserve may cause respiratory failure. Pacemakers and pacemaker wires, which can conduct electrical current from radiofrequency and, to a lesser degree, microwave energies along the pacemaker wires can result in application of thermal energy in unintended remote locations. Additionally, a pacemaker may malfunction during RFA. Patients with pacemakers can still receive RFA provided the pacemaker is turned off prior to the procedure. 14
3.5 Preprocedure Workup
Preprocedural evaluation of a patient typically includes a patient history, with focus on cardiopulmonary status, pulmonary infection, bleeding diatheses, and medications. Platelet count and coagulation profile are essential. Pulmonary function tests should be reviewed to check for adequacy of oxygenation, pulmonary reserve, flow-volume spirometry, and fitness for sedation and lung reduction, particularly in those patients with lung disease or prior resection.
The presence of a pacemaker or automatic implantable cardioverter defibrillator (AICD), especially in a patient with constant dependence, should increase consideration of PCT and MWA, rather than RFA, which can cause device malfunction. Alternatively, if RFA is the preferred ablative technique, and the patient has limited dependence on a cardiac pacer, a deactivated pacemaker with transvenous or temporary external cardiac pacing is an acceptable strategy.
A thorough medication history should be obtained with attention to anticoagulant and antiplatelet medications, which should be stopped or tapered prior to the anticipated procedure. Conversion to subcutaneous or low molecular weight heparin can be considered for those patients on oral anticoagulants, which can be stopped at least 24 hours prior to the procedure.
Histopathologic diagnosis of the lesion(s) should be obtained before the ablation. Biopsy can be performed prior to the ablation as a separate session or immediately prior to the ablation as a concurrent session. At our institution, we prefer to perform the percutaneous biopsy approximately 1 week prior to the ablation to allow for perilesional hemorrhage to resolve, to confirm the final pathology, and for clear tumor margins to be apparent at the time of the ablation. Furthermore, longer procedure times, or a delayed or postponed ablation procedure related to biopsy complications, may result when biopsy occurs prior to ablation as a concurrent session.
There is no standardized agreement on the pre- or postprocedural administration of antibiotics. At our institution, we typically do not give antibiotics for most chest ablations.
Ideally, a preprocedure chest CT scan should be performed within 4 weeks of the anticipated procedure to assess (1) tumor size and number; (2) lesion shape and location in relation to vessels, bronchi, and other vital structures; (3) the presence of comorbid lung disease, including advanced bullous disease, radiation injury, or infection; and (4) a safe percutaneous access route.
PCT of the chest is typically performed under conscious sedation, although general anesthesia is at times warranted during ablations that require a higher degree of monitoring. No matter the choice of anesthesia employed during thermal ablation of the chest, it must be efficient at both controlling pain and maximizing the reproducibility of the target tumor position to facilitate accurate probe placement. Additionally, there is an increased risk of pneumothorax from positive pressure ventilation with general anesthesia. 15 For these reasons, most experienced operators prefer conscious sedation in nearly all percutaneous thermal ablations of the thorax.
On occasion, general anesthesia is preferred, including in cases with increased risk of bleeding, in patients with tenuous respiratory status, or to allow for selective intubation of a bronchus to improve positioning of the contralateral-targeted pulmonary lesion in relation to vital structures. The most common indication for the escalation of sedation level to general anesthesia is when using RFA for treatment of chest wall and pleural lesions, a procedure that is often quite painful. 9
3.6 Selection Criteria
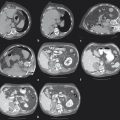
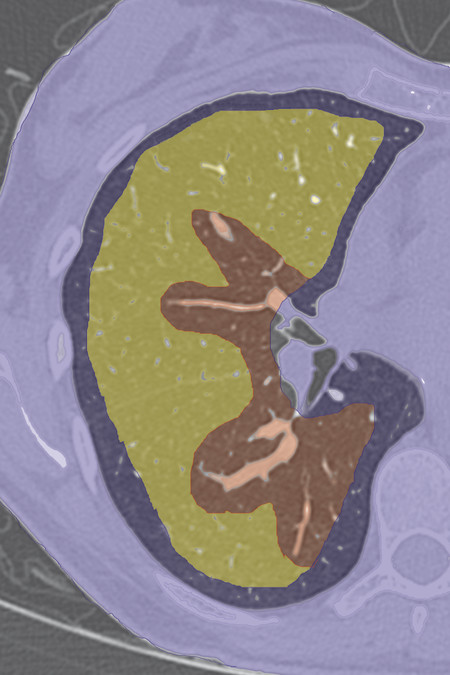
Studies have repeatedly identified certain lesion characteristics for which ablation can be expected to achieve a complete result of total tumor kill without recurrence ( Table 3.1 and Fig. 3.2 ). These include size, location, number of lesions, and adjacent structures.
Comparative technologies | |||
Parameter(s) | Radiofrequency | Microwave | Cryoablation |
< 3 cm | +++ | +++ | +++ |
> 3 cm | + (up to 3)a | +++ (up to 3)a | ++ (up to 20)a |
≤ 1.5 cm pleura | + (pain) | + (air leak) | +++ |
Chest wall and pleura | + | ++ | +++ |
Mediastinum | + | + | ++ |
Sinks (heat/cold) | + | +++ | ++ |
Pacer and AICD | + | ++ | +++ |
Coagulopathy | +++ | +++ | + |
Maneuverability | (+) | (+) | + |
Abbreviation: AICD, automatic implantable cardioverter defibrillator. aThe number of simultaneous applicators for each respective therapeutic modality. | |||
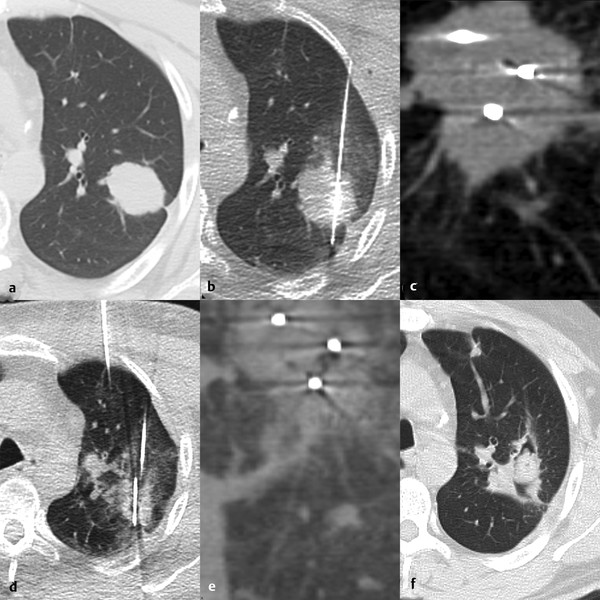
3.6.1 Size
Lesions < 3 to 3.5 cm 13 , 16 are more likely to be fully treated than larger lesions. Multiple RFAs and cryoprobes are used for lesions > 2 cm. MWA is preferred for larger lesions, with its ability to treat up to 3.5 cm ( Fig. 3.3 ) and possibly larger lesions.
3.6.2 Location
For completely intraparenchymal lesions, any of the three percutaneous ablative therapies can be used. For juxtapleural, pleural, and chest wall lesions, cryoablation is preferred to the heat-based ablative therapies due to less patient discomfort and fewer complications. 17
3.6.3 Number of Lesions
For metastatic disease, there are ideally fewer than six pulmonary lesions, which is loosely based on metastasectomy data showing decreased survival with an increasing number of resections. 18
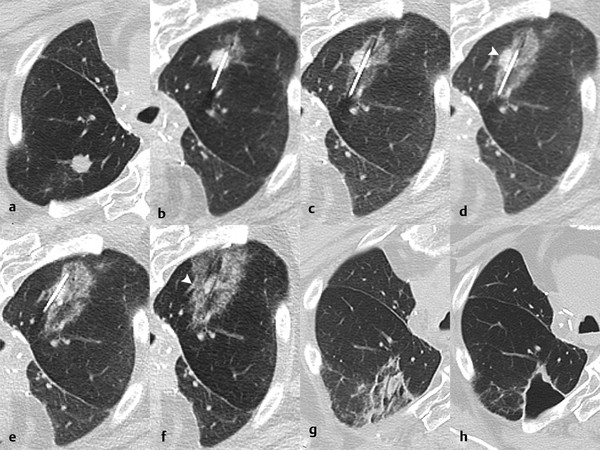
3.6.4 Adjacent Structures
Ablation adjacent to a pulmonary artery or vein measuring > 3 mm, 19 , 20 or large bronchus, 21 using RFA or PCT, may result in incomplete ablation margins due to heat sink or cold sink, respectively. MWA reduces, but does not completely negate, this heat sink phenomenon 10 ( Fig. 3.4 ).
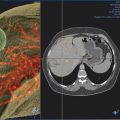
PCT is preferred for lesions adjacent to the hilum and vital mediastinal structures, such as the aorta, heart, esophagus, and trachea, due to the preservation of the tissues’ collagenous architecture and visualization of ice ( Fig. 3.5 ). In some situations, the ability to “stick” the cryoprobe to the tumor allows the targeted tissue to be torqued away from adjacent structures.
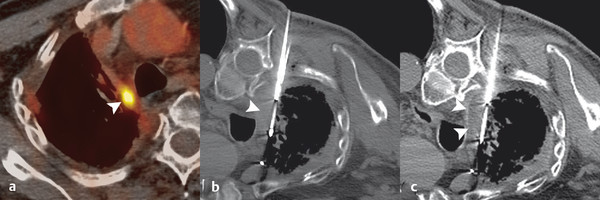
Similarly, cryoablation is preferred for pleural and chest wall lesions because it results in less pain and tissue injury as compared to RFA and MWA, respectively.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree