Principles of stem cell transplantation
Stem cell transplantation (SCT) involves eliminating a patient’s haemopoietic and immune system by chemotherapy and/or radiotherapy and replacing it with stem cells either from another individual or with a previously harvested portion of the patient’s own haemopoietic stem cells (Fig. 23.1). The term encompasses bone marrow transplantation (BMT), in which stem cells are collected from bone marrow, peripheral blood stem cell (PBSC) transplantation and umbilical cord stem cell transplantation.
Figure 23.1 Procedures for (a) allogeneic, and (b) autologous stem cell transplantation. G-CSF, granulocyte colony-stimulating factor; GVHD, graft-versus-host disease; HLA, human leucocyte antigen. For non-myeloblative (reduced intensity) allogeneic SCT, lower doses of chemotherapy with or without radiotherapy are used.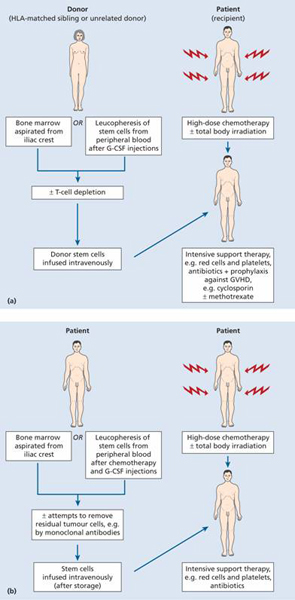
SCT may be syngeneic (from an identical twin), allogeneic (from another person) or autologous (from the patient’s own stem cells) (Table 23.1).
The principal diseases for which SCT is performed are listed in Table 23.2. However, the exact role of SCT in the management of each disease is complex and depends on factors such as disease severity and subtype, remission status, age and, for allogeneic transplantation, availability of a donor.
Table 23.2 Stem cell transplantation: indications.
| Allogeneic (or syngeneic) | Autologous |
| Acute lymphoblastic or myeloid leukaemia. Other malignant disorders of the marrow (e.g. chronic myeloid leukaemia, myelodysplasia, multiple myeloma, lymphoma, severe aplastic anaemia including Fanconi’s anaemia) | Hodgkin lymphoma and non-Hodgkin lymphoma, multiple myeloma, AML, primary amyloidosis |
| Inherited disorders: thalassaemia major, sickle cell anaemia, immune deficiencies, inborn errors of metabolism in the haemopoietic and mesenchymal system (e.g. osteopetrosis) | |
| Other acquired severe marrow diseases (e.g. paroxysmal nocturnal haemoglobinuria, red cell aplasia, myelofibrosis) |
AML, acute myeloid leukaemia.
Collection of stem cells
Stem cells can be collected from the peripheral blood, bone marrow or umbilical cord blood.
Peripheral blood stem cell collection
PBSCs are taken using a cell-separator machine connected to the patient or donor via peripheral cannulae (Fig. 23.2). Blood is taken through one cannula and pumped around the machine where mononuclear cells are collected by centrifugation before the red cells are returned to the patient. This continuous process may take a few hours before enough mononuclear cells are collected.
Figure 23.2 Peripheral blood stem cell (PBSC) collection: a donor undergoing collection of PBSCs on a cell separator.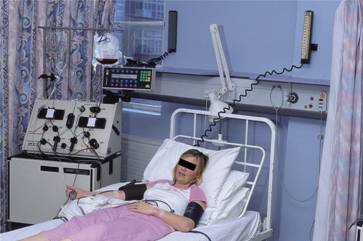
Peripheral blood normally contains too few haemopoietic stem cells to allow collection of sufficient numbers for transplantation. Chemotherapy and growth factors can each increase the number by around 10 – 100 times. Chemotherapy is used in patients undergoing autologous stem cell collection but not in healthy donors. PBSCs are usually collected during the recovery phase from a cycle of chemotherapy (e.g. 1.5 g/m 2 cyclophosphamide). In future it is possible that stem cell populations may also be expanded in vitro.
Granulocyte colony-stimulating factor (G-CSF) is given to patients or donors as a course of injections (typically 10 μ g/kg/day for 4 – 6 days) until the white cell count starts to rise. PBSC collections are then taken and, depending on the efficiency of stem cell mobilization, repeated collections may be needed for up to 3 days. The adequacy of the collection may be assessed by:
1 CD34+ cell count. Generally >2.0 × 106/kg are needed for transplantation.
2 In vitro colony assays, particularly granulocyte–macrophage colony-forming unit (CFU-GM) (see p. 3), of which 1–5 × 105/kg would be considered adequate for transplantation.
Bone marrow collection
The donor is given a general anaesthetic and 500–1200 mL marrow is harvested from the pelvis. The marrow is anticoagulated and a mononuclear cell count is taken to assess the yield, which should be approximately 2–4 × 108 nucleated cells/kg body weight of the recipient.
Umbilical cord blood
Fetal blood is a rich source of haemopoietic stem cells which may be collected from cord blood. Because of the relatively small numbers of stem cells collected from a single cord, they are most useful for children who do not have a fully matching sibling or unrelated donor. Less stringent human leucocyte antigen (HLA) matching is needed. Double cord donations may be needed to obtain sufficient stem cells for adult recipients.
Stem cell processing
After collection the stem cell harvest can be processed with removal of red cells and concentration of the mononuclear cells. Autologous collections may be ‘purged’ by chemotherapy or antibodies in an attempt to remove residual malignant cells. Allogeneic collections may be treated with antibodies to remove T cells to reduce graft-versus-host disease (GVHD). CD34+ stem cells may be selected from both types of harvest (Fig. 23.3).
Figure 23.3 Peripheral blood stem cell collection: enriched CD34 + cells stained by May – Gr ü nwald-Giemsa stain. The cells have the appearance of small-and medium-sized lymphocytes.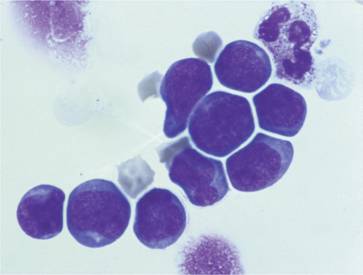
Conditioning
Prior to infusion of haemopoietic stem cells patients receive chemotherapy, sometimes in combination with total body irradiation (TBI; Fig. 23.1) in a procedure called conditioning. This is designed to eradicate the patient’s haemopoietic and immune system and, if present, malignancy. In addition, in the setting of allogeneic SCT, by suppressing the host immune system it helps to prevent rejection of the ‘foreign’stem cells. An important development that has occurred in SCT is a major shift from myeloablative regimens to non-myeloablative conditioning.
Myeloablative conditioning regimens irreversibly destroy the haemopoietic function of the bone marrow with high doses of chemotherapy or radiotherapy. TBI is usually used in patients with malignant disease and is administered as a single dose or in smaller doses over several days (fractionated). The most commonly used chemotherapy drug is cyclophosphamide but busulfan, melphalan, cytosine arabinoside, etoposide or nitrosoureas are given in some protocols. At least 36 hours are allowed for the elimination of the drugs from the circulation following the last dose of chemotherapy before donor stem cells are infused. Conditioning therapy is often complicated by mucositis and patients sometimes need parenteral nutrition. Trials are taking place in which monoclonal antibodies directed against specific antigens such as CD45 are attached to toxins or radioactive isotopes in an attempt to selectively target white cells as an aid to conditioning.
Non-myeloablative conditioning regimens have been developed to reduce the morbidity and mortality of allogeneic transplantation and do not completely destroy the host bone marrow. These can include agents such as fludarabine, low-dose irradiation, antilymphocyte globulin or other antibodies that delete T cells, and low doses of busulfan or cyclophosphamide. The aim in these ‘mini-or reduced-intensity-transplants’ is to use enough immunosuppression to allow donor stem cells to engraft without completely eradicating host marrow stem cells. Donor leucocyte infusions (DLI) are commonly used at a late stage in order to encourage complete donor engraftment. Such regimens extend the age range and increase the treatment indications for allogeneic transplantation.
Post-transplant engraftment and immunity
After a period of typically 1–3 weeks of severe pancytopenia, the first signs of successful engraftment are monocytes and neutrophils in the blood with a subsequent increase in platelet count (Fig. 23.4). A reticulocytosis also begins and natural killer (NK) cells are among the earliest donor-derived lymphocytes to appear. G-CSF may be used to reduce the period of neutropenia. Engraftment is usually quicker following PBSC transplantation than BMT.
Figure 23.4 Typical haematological chart of a patient undergoing allogeneic marrow transplantation for aplastic anaemia. WBC, white blood cells.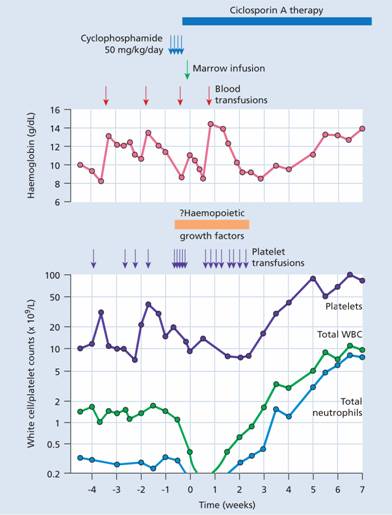
The marrow cellularity gradually returns to normal but the marrow reserve remains impaired for 1–2 years. There is profound immunodeficiency for 3–12 months with a low level of CD4 helper cells and a raised CD8: CD4 ratio for 6 months or more. Immune recovery is quicker after autologous and syngeneic SCT than following allogeneic SCT. The patient’s blood group changes to that of the donor and antigen-specific immunity becomes that of the donor after approximately 60 days.
Autologous stem cell transplantation
This allows the delivery of a high dose of chemotherapy, with or without radiotherapy, which otherwise would result in prolonged bone marrow aplasia. Stem cells are harvested and stored before the treatment is given and are then reinfused to ‘rescue’ the patient from the myeloablative effects of the treatment (Fig. 23.1). A limitation of the procedure is that tumour cells contaminating the stem cell harvest may be reintroduced into the patient. Nevertheless, autografting has a major role in the treatment of haematological diseases such as lymphoma and myeloma. The major problem associated with autografting is recurrence of the original disease. GVHD is not an issue. Procedure-related mortality is generally well below 5%.
Allogeneic stem cell transplantation
In this procedure, stem cells harvested from another person are infused into the patient. The procedure has a significant morbidity and mortality and one of the major reasons is the immunological incompatibility between donor and patient despite HLA matching. This may manifest as immunodeficiency, GVHD or graft failure. Paradoxically, there is also a graft-versus-leukaemia (GVL) effect which probably underlies much of the success of the procedure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree