2 Adrenal Malignancy: Ablation
2.1 Introduction
Adrenal masses are a common clinical challenge, and incidental adrenal lesions may be detected on imaging in up to 10% of the general population. 1 , 2 , 3 Most adrenal incidentalomas are benign, with the nonfunctioning adrenal adenoma representing 80% of all incidentally detected adrenal neoplasms. 1 , 2 However, the possibility of malignant disease is the major concern when an incidental mass is identified, making biopsy or definitive diagnosis requisite prior to many ablations. 1 , 2 Malignancies of the adrenal gland include adrenal metastases as well as primary tumors, such as adrenocortical carcinoma, malignant pheochromocytoma, and neuroblastoma. Because the adrenal gland is the fourth most common site of metastatic disease, metastases to the adrenal gland are readily encountered. Primary tumors with a propensity to metastasize to the adrenal gland include lung, renal, and gastrointestinal cancers and melanoma. 4 Adrenocortical carcinoma, a rare but aggressive malignancy originating in the adrenal cortex, has an incidence of 1 to 2 per million population annually. 5 Early metastases are common, with overall 5-year survival being 20 to 35%. 6 Pheochromocytoma, a neuroendocrine tumor arising from the chromaffin cells of the adrenal medulla, can also pose a challenge for standard surgical or nonsurgical palliative management in patients with metastatic or recurrent disease. 7 , 8 , 9 Patients with metastatic pheochromocytoma may present with painful lesions, life-threatening lesions, or symptoms related to these catecholamine-producing neuroendocrine tumors. 7 , 8 , 9
Surgical resection remains the mainstay of therapy for localized primary adrenal malignancies. Adrenal metastases in the setting of disseminated disease are conventionally treated with systemic chemotherapy. 4 There is some evidence to suggest that, in selected cases, surgical resection of isolated adrenal metastases may be associated with improved survival, although this remains an area for ongoing investigation. 4 , 10 , 11 , 12 The need for alternative therapies in nonsurgical adrenal cancer patients and the increased detection of adrenal tumors with cross-sectional imaging has prompted application of percutaneous ablative therapies for the management of adrenal malignancy and metastatic cancers of adrenal origin. 4 Both percutaneous thermal and chemical ablative techniques have been used in the treatment of adrenal malignancy, although chemical ablation has not achieved desirable complete response rates, despite excellent results for treatment of benign functional adrenal tumors. 13 This chapter summarizes the role of percutaneous thermal ablation, including radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation, in the management of adrenal malignancy, with an emphasis on clinical and technical considerations unique to the adrenal gland.
2.2 Indications
Percutaneous thermal ablation of adrenal malignancy has been performed in patients with adrenal metastases, locally recurrent or metastatic adrenocortical carcinoma, and pheochromocytoma metastases who are considered unresectable or poor surgical candidates, who have undergone previous attempts at surgical debulking, and who refuse surgery. 14 , 15 Determination of the potential benefit from ablation and its impact on survival or quality of life is ideally determined by multidisciplinary consensus.
2.3 Contraindications
Uncorrectable coagulopathies and hemorrhagic diatheses are relative contraindications to percutaneous ablation for adrenal malignancy. Blood product use may be employed as for surgical procedures. 16 The international normalized ratio should ideally be < 1.5 to 1.8 and the platelet count ≥ 50 × 109/L prior to the procedure, 15 although some use 75 × 109 instead of 50 × 109/L as a threshold. Ablation should not be performed in those patients who are acutely ill or septic. Comorbid conditions, such as chronic obstructive pulmonary disease and congestive heart failure, are not contraindications to ablation; however, multiple comorbid conditions may increase the risk profile. Prior hypertensive crisis or elevated levels of catecholamines also raise the periprocedural risks but are not absolute contraindications with appropriate precautions. 16
2.4 Patient Selection and Preprocedure Workup
The management of adrenal malignancies requires a multidisciplinary approach, including involvement from medical, surgical, and radiation oncology; endocrinology; and interventional radiology. 4 The patient’s clinical and treatment history, lesion histology, and imaging findings should be reviewed followed by a discussion of available treatment options, including surgery, radiation, chemotherapy, and ablative therapy. Before proceeding with ablation, all specialists should ideally be in agreement regarding the utility of ablation, with or without additional medical or surgical treatments. 4 When appropriate, the risk of hypertensive crisis and specific medical risks in patients with significant comorbidities should be discussed. Preprocedure cross-sectional imaging should be reviewed in detail for tumor size, location, and potential access routes or proximities of adjacent organs. 15 Laboratory data, particularly coagulation parameters, should be reviewed for procedural eligibility. For suspected functioning tumors, appropriate serum or urine assays for cortisol, aldosterone, and catecholamines may be obtained before ablation to assess their interval change after treatment. 15
In general, preablation tumor biopsy is typically recommended because it can prevent unnecessary treatment and more accurately report ablation efficacy. 14 , 17 Additional factors in favor of pretreatment biopsy include patient preference for a definitive diagnosis, the fact that ablation does not yield a resection specimen, and the need to inform follow-up imaging planning. 17 When planning biopsy for an unknown adrenal tumor, it is important to be prepared for the risk of hypertensive crisis, particularly if the lesion suspected is a pheochromocytoma, for which intraprocedural anesthesia monitoring and readiness are recommended to enable acute management of hypertension. If clinical history and diagnostic imaging suggest pheochromocytoma, prebiopsy plasma or urinary catecholamines are helpful; positive catecholamines may obviate biopsy or indicate the need for arterial pressure monitoring, administration of alpha (α)- and beta (β)-adrenergic inhibitors, or anesthesia consultation if biopsy is to be pursued. 15 When the clinical diagnosis of an adrenal mass can be made confidently on the basis of clinical history, imaging, and endocrinologic findings, biopsy may be avoided; however, evaluation should be on a case-by-case basis. 14
Preablation endocrinology consultation is critical in the workup of adrenal tumors. The adrenal gland’s unique endocrine properties include the secretion of catecholamines (epinephrine and norepinephrine) by the adrenal medulla. 18 As a result, the interventional oncologist must be mindful of the risk of intraprocedural hypertensive crisis during ablation of intra-adrenal malignancies or metastases from functional adrenal malignancies like pheochromocytoma. 14 , 15 , 19 , 20 Of note, hypertensive crisis has been observed, even during ablation of tumors in proximity to the adrenal gland. 21 Preablation endocrinology consultation ensures appropriate management of functional adrenal tumors to minimize the risk of intraprocedural hypertensive crisis as well as to assist with postprocedure monitoring of hormone levels and replacement therapy, as needed. 15 Preablation antihypertensive regimens may include a combination of α- and β-adrenergic inhibitors, which may be titrated for 7 to 21 days prior to the procedure, depending on patient blood pressures ( Table 2.1 ). When one is premedicating patients for planned adrenal ablation, it is important to note that β-adrenergic inhibitors should not be administered as a sole agent. 4 To minimize the risk of hypertensive crisis and heart failure secondary to unopposed α-adrenergic stimulation α-adrenergic inhibition with phenoxybenzamine should be initiated before β-adrenergic inhibitors. 4 Following several days of phenoxybenzamine to achieve sufficient α-adrenergic inhibition, β-adrenergic inhibition can be performed with an agent such as oral atenolol or labetalol. In selected cases prior to metastatic pheochromocytoma ablation, our endocrinologists have also recommended administration of the tyrosine hydroxylase inhibitor α-methyl-paratyrosine to inhibit ongoing catecholamine synthesis before the procedure. If there are adjustments to patients’ medication regimens in anticipation of ablation, it is important to ensure follow-up during this time by a clinical practitioner, so that patients may be screened for signs and symptoms of orthostasis, and counseled about the risks of dizziness, falls, or other side effects. In select patients, it may be of value to intentionally titrate medications such that the patient is orthostatic preprocedurally, in order to better tolerate catecholamine release during subsequent ablation ( Table 2.1 ).
Preablation anesthesia consultation is also advisable, given the possibility of intraprocedural catecholamine release and hypertensive crisis during ablation of adrenal malignancy. General endotracheal anesthesia may be warranted, with arterial blood pressure monitoring during pheochromocytoma ablation. 9 Careful attention to pre- and intraprocedural medication management and medical monitoring is especially critical in the context of suspected or confirmed pheochromocytoma. Catastrophic clinical consequences of hypertensive crisis and cardiac arrest have been reported during pheochromocytoma metastasis ablation performed in the absence of preprocedural α-adrenergic inhibition, despite intraprocedural anesthesia monitoring. 22 Anesthesiologist readiness with premixed, ready to inject (or drip) short-acting beta-blocker (e.g., esmolol) plus a titratable antihypertensive (e.g., nitroprusside) is advisable. A bag compression device for rapid administration of nitroprusside without delay may be a worthwhile precaution. Careful communication with the anesthesiologist when turning on or off ablative energies is critical and allows adequate preparation. RFA and MWA can be turned off during ablation in the event of hypertension, and there is often a latency period of approximately 30 to 60 seconds between the termination of RF current and the peak blood pressure. It is unknown whether ice ball thawing during cryoablation corresponds temporally with catecholamine release.
2.5 Technique
2.5.1 Methods of Ablation
Percutaneous ablation of adrenal malignancy has been performed using RFA, MWA, and cryoablation.
Radiofrequency Ablation
RFA typically employs three equipment components 1 : a radiofrequency generator generating an alternating current in the 500 kHz range 2 ; single- or multitined needle electrodes, which may be monopolar or bipolar; and, in the case of monopolar needle electrodes, 3 grounding pads, which are placed on the patient’s thighs. The needle electrode is placed within the tumor by the operating physician, typically under ultrasound and/or computed tomography (CT), cone beam CT (CBCT), or CT/fluoroscopy guidance. After connecting the needle electrode and grounding pads to the generator, a closed loop circuit is created with the patient’s target tumor serving as the end resistor. Radiofrequency energy applied to the needle electrode by the generator results in the generation of ionic agitation at the tip of the needle electrode, leading to frictional heat and near instantaneous coagulation necrosis of tissue when temperatures between 60 and 100°C are achieved. For adequate destruction of tumor tissue, the targeted volume must be treated with temperatures that are above the 60°C threshold for cell death. 14 , 23 Ablation zones will typically be limited to the region of tissue immediately surrounding the electrode, with most RFA devices generating an approximately 3 cm single ablation zone. To treat a larger tumor, multiple ablations are overlapped to generate a composite ablation zone of sufficient diameter to encompass the tumor and a surrounding tumor-free margin. 24 Real-time ultrasound visualizes gas within the treated region during ablation, which is an estimate of ablation, although less accurate compared to CT or magnetic resonance imaging (MRI). When employing CT guidance, iodinated contrast may be administered at the end of the treatment to assess the expected nonenhancement of the ablation zone.
The companies in the United States manufacturing RFA devices are AngioDynamics (Queensbury, NY), Boston Scientific (Natick, MA), Covidien (Mansfield, MA), and RFA Medical, Inc. (Fremont, CA). 25 Generators are programmed to generate several ablative cycles. The length of the ablation cycle and number of cycles applied to a tumor are dependent on the desired size of the ablation zone. In general, most systems apply an initial gradual increase in power, and once peak power is achieved, it is held for a 12- to 15-minute ablation cycle until energy output feedback or elevations in tissue impedance stop the generator current flow and ablation. 25 For catecholamine-secreting pheochromocytomas, although the rationale is anecdotal, it has been our practice to slowly increase the electrical current at the beginning of an ablation, in order to better assess the degree of resultant hypertension, rather than immediately applying maximal current. In this way, the RFA can be reduced or switched off during episodes of marked hypertension. This technique may permit safer application of RFA in this setting; however, patience is also required because this approach may prolong overall treatment time.
Microwave Ablation
Microwave ablation involves transmission of microwaves through an antenna inserted into a tumor. With application of an oscillating electromagnetic field of 915 or 2,450 MHz in frequency, ionic agitation of water molecules adjacent to the tip of the microwave antenna results in frictional agitation heat and cell death on the basis of coagulative necrosis. 25 In contrast to RFA, grounding pads are not required, given the inherent nonelectric properties of the electromagnetic wave. 14 As is the case for RFA, real-time imaging with ultrasound may be used, which enables visualization of ultrasound echogenicity secondary to the generation of gas within the treated region during ablation. Iodinated contrast may be administered following treatment to assess the size and geometry of the ablation zone. Analogous to RFA, the targeted volume must be treated with temperatures that are above the 60°C threshold for cell death for adequate destruction of tumor tissue. Real-time intraprocedural information about ablation zone temperatures is obtained from thermocouple readings and the manufacturer’s information about probe heating capabilities. 25 Compared to RFA, MWA results in faster heating over a larger volume with less susceptibility to heat sink or local perfusion. Higher temperatures and larger ablation zones are therefore achieved in a shorter period of time. 25 , 26 , 27 , 28 Use of MWA over RFA may be particularly advantageous for large tumors, such as those ≥ 5 cm in diameter. 29
The MWA systems commercially available in the United States employ either a 915 MHz generator (Evident, Covidien; Avecure, Medwaves) or a 2,450 MHz generator (e.g., Certus 140, Neuwave; Amica, Hospital Service; Acculis MTA, Angiodynamics). The antennae used are straight applicators with active tips, ranging in lengths from 0.6 to 4 cm. The majority of available systems require that the antennae are internally cooled with either room-temperature fluid or carbon dioxide to reduce conductive heating and prevent thermal injury to the skin. 30 Although MWA has been associated with oblong or ellipsoid treatments, including proximal burns along the probe shaft, recent technology makes spherical ablations more possible.
Cryoablation
Cryoablation makes use of the Joule–Thomson effect, which describes the change in temperature of a gas resulting from expansion or compression of that gas. 31 Expansion of argon inside a small chamber within the end of a cryoprobe results in cooling of the gas prior to tissue exposure. 25 Extremely low temperatures are produced when an active cryoprobe is placed into a tumor. The application of a series of alternating freeze–thaw cycles results in the generation of intra- and extracellular ice crystal formation and cell membranolysis. Cell death occurs on the basis of apoptosis, with temperatures of − 20 to − 40°C necessary for cell death. Cryotherapy also results in small-vessel thrombosis, which can contribute to indirect cell destruction. 25 Cryoprobes are placed into the target tumor under ultrasound, CT, CBCT, CT/fluoroscopy, or MRI guidance. If multiple probes are placed within a tumor, they are ideally placed within 1 to 2 cm of each other and within 1 cm of the tumor margin. 25 The ice ball generated during the freeze cycles may be visualized as a well demarcated echogenic near-field region by ultrasound, as an area of hypodensity on CT, or as a region of signal loss on MRI. This finding can be used to estimate adequacy of probe geometry and tumor coverage, although the ablation margin may be ~ 4 mm within the circumference of the ice ball. Visualization of the ice ball generated during treatment may also obviate the need for intraprocedural contrast administration. Analogous to RFA, cryoablation shows relative sparing of damage to larger vessels, which can result in heat-sink (in this case cold-sink) effects, necessitating colder thermal temperatures to cause cell death. Potential complications related to the lack of coagulative necrosis achieved with cryoablation include the potential for increased risk of bleeding compared with hyperthermic technologies. In addition, tumor lysis syndrome or cryoshock can occur, attributed to exposure of ablation zone contents to the systemic circulation. 32 Two companies in the United States currently market commercial cryoablation systems for percutaneous use, the Percryo device, (Healthtronics, Inc.) and the Presice and SeedNet systems, manufactured by Galil Medical. 25 These systems employ argon, helium, or nitrogen gas for cooling. The probes provided with these systems range from 1.4 to 4.9 mm in diameter and can achieve a range of ablation zone sizes. 25
Technical Considerations
Imaging Guidance
Thermal ablation of adrenal neoplasms may be performed with CT fluoroscopy guidance, CT guidance, CBCT, or with a combination of ultrasound and CT guidance. 33 , 34 MRI may also be used for intraprocedural imaging, but it has limited widespread availability and requires a special system, often with MRI room modifications. Advantages of CT guidance include visualization of the targeted mass and the location and delineation of vulnerable regional anatomy. Concomitant fluoroscopy is particularly advantageous for real-time visualization of the hemidiaphragms during needle insertion to avoid transpleural transgression. Ultrasound alone may not reliably identify the adrenal gland, although it can have value for real-time monitoring during ablation of large adrenal tumors. 33 , 34 CBCT with integrated real-time fluoroscopic guidance can combine several advantages, but it may have a limited field of view and may complicate the intraprocedural use of ultrasound.
Patient Positioning
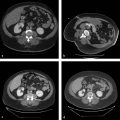
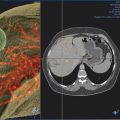
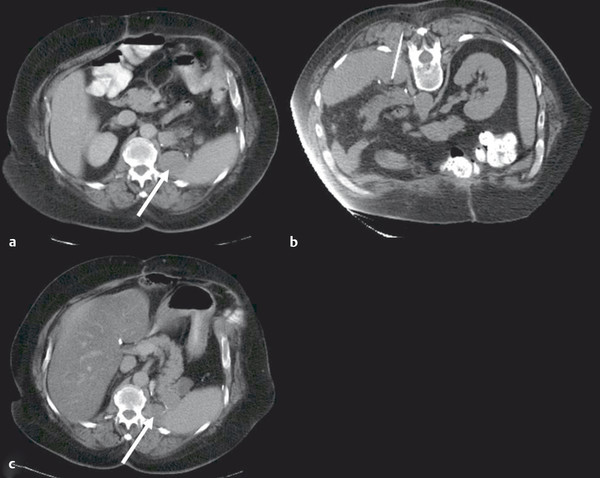
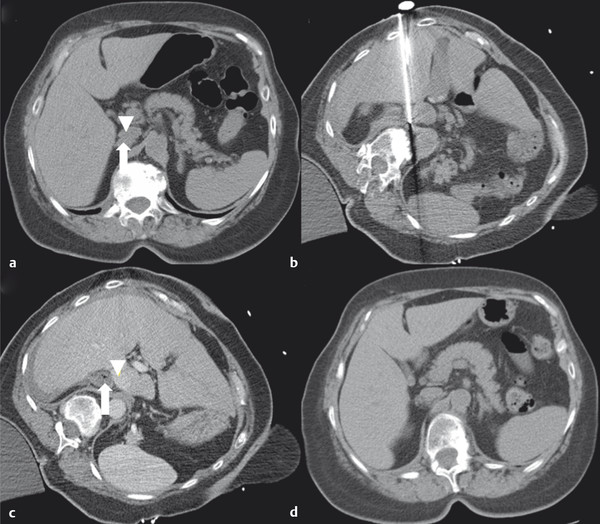
At the onset of percutaneous ablation of a primary or metastatic adrenal tumor, the access or approach to the lesion should be assessed on preablation imaging. The location of vulnerable regional anatomy should be evaluated at this time, and the need for protective maneuvers, such as hydrodissection, should be evaluated. Commonly vulnerable anatomical structures during adrenal ablation include the lung and pleura, kidney, colon, duodenum, stomach, pancreas, liver, and spleen. 4 , 34 Decubitus positioning of the patient (target side down) during adrenal ablation is particularly useful to gain access to the adrenal gland while avoiding transpleural transgression. In the case of large tumors, prone positioning may facilitate safe access to the target tumor, if transpleural transgression is avoidable ( Fig. 2.1 ). For cases when the adrenal tumor cannot be successfully approached via a posterior approach due to intervening lung parenchyma or the presence of colon, an anterolateral, transhepatic approach may be useful ( Fig. 2.2 ). 15
Intraprocedural Monitoring and Adjunctive Maneuvers
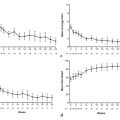
Careful intraprocedural monitoring is critical for safe adrenal tumor ablation. Hypertension can occur during adrenal ablation as a result of catecholamine release. Preclinical studies have demonstrated that RFA of normal adrenal tissue may cause intraprocedural hypertension, with elevations in both norepinephrine and epinephrine during porcine adrenal RFA and elevations in norepinephrine during canine adrenal RFA. 35 , 36 In a review of five reports, including more than 40 patients undergoing adrenal RFA for both benign functional adrenal neoplasms and adrenal malignancy, a range of incidences of intraprocedural hypertension has been described, from 0–66.7%. 34 , 35 , 37 , 38 , 39 Moreover, a significantly higher rate of intra-procedural hypertension has been described in patients undergoing (nonpheochromocytoma) adrenal tumor ablation (6 of 9, 66.7%) compared to nonadrenal abdominal tumors (1 of 9, 11.1%). 35 In a study of 9 patients undergoing adrenal RFA, intraprocedural systolic blood pressure correlated significantly with serum epinephrine (R2 = 0.68, P < 0.0001) and norepinephrine values (R2 = 0.72, P < 0.0001), whereas other adrenal hormones (dopamine, cortisol) did not correlate with systolic blood pressure. 35 Although rare, hypertensive crisis has also been reported during RFA of hepatic tumors in proximity to the adrenal gland. 19 Etiologic factors implicated for intraprocedural hypertension during adrenal ablation include pain-and anxiety-mediated release of catecholamines, rapid release of catecholamines during destruction of normal adrenal tissue, and RFA-mediated electrical stimulation resulting in catecholamine release. 35 A prior case report has also described malignant hypertension during cryoablation of an adrenal gland tumor, presumably based on analogous mechanisms. 20
Significant elevations in intra-arterial systolic blood pressure (> 200 mm Hg) and diastolic blood pressure (> 100 mm Hg) have also been observed, specifically in the setting of thermal ablation of pheochromocytoma metastases. 9 Analogous to physical manipulation of these tumors in the operating room, ablation-related physical and thermal stimuli may potentiate tumor catecholamine release, causing abrupt elevations in patient heart rate and blood pressure. 9 During ablation of pheochromocytoma metastases, close communication with the anesthesiologist is critical, with announcements prior to probe insertion into tumor, application of thermal energy, and probe repositioning and removal. Although unusual, needle removal itself can result in immediate hypertension. Gradual, manual application of thermal energy and incremental increases in the energy delivered have been advocated to allow adequate time to observe hemodynamic changes and for careful titration of medication, as needed. 9 General anesthesia with intra-arterial blood pressure monitoring should be considered prior to thermal ablation of these tumors. A combination of β-adrenergic antagonists (e.g., esmolol, labetalol, metoprolol), phenoxybenzamine, sodium nitroprusside, nitroglycerine, and analgesia/anesthetics (e.g., fentanyl, hydromorphone, midazolam, propofol) has been successfully used for intraprocedural control of hypertension, with authors advocating target blood pressures and heart rates within 20% of the patient’s baseline hemodynamic profile. 9 Table 2.1 presents suggested pre-and intraprocedural medications and dosages for pheochromocytoma ablation, although it remains vital for the interventional radiologist to evaluate each patient on a case-by-case basis and in conjunction with consulting endocrinology and anesthesiology services when determining optimal medical management in these cases. Pheochromocytoma ablation can be a challenging procedure for the operating physician due to the rapid or extreme fluctuations in hemodynamics.
Suggested preprocedural medication regimen | |||||
Medication class | Suggested agent | Dose | Initiation | Duration | Comments |
α-adrenergic inhibitor | Phenoxybenzamine | 10 mg 2–3 times/d | 7–14+ days prior to ablation | Continue up to treatment day | Titrate following ablation depending on hemodynamic response |
β-adrenergic inhibitor | Atenolol | 12.5 mg twice daily | 7–14+ days prior to ablation | Continue up to treatment day | Titrate following ablation depending on hemodynamic response |
Dopamine synthesis inhibitor (tyrosine hydroxylase inhibitor) | α-methyl-para-tyrosine | 250 mg 2–3 times/d | 7–14+ days prior to ablation | Continue up to treatment day | Titrate following ablation depending on hemodynamic response |
Suggested intraprocedural medications | |||||
Medication class | Agent | Dose | Comments | ||
α-adrenergic inhibitor | Phenoxybenzamine | IV administration | Administration and titration per intraprocedural hemodynamic response | ||
β-adrenergic inhibitor | Labetalol, esmolol, metoprolol | IV administration | Administration and titration per intraprocedural hemodynamic response | ||
Nitric oxide–based vasodilator | Sodium nitroprusside, nitroglycerin | IV administration | Administration and titration per intraprocedural hemodynamic response | ||
Opioid analgesic | Fentanyl, hydromorphone, | IV administration | Administration and titration per intraprocedural hemodynamic response | ||
Hypnotic/amnestic | Propofol | IV administration | Administration and titration per intraprocedural hemodynamic response | ||
Benzodiazepine, short acting | Midazolam | IV administration | Administration and titration per intraprocedural hemodynamic response | ||
Abbreviation: IV, intravenous. Note: It is critical to note that the agents, their timing, dosage, and duration of administration presented in this table are suggestions only. Interventional radiologists are urged to consult members of their endocrinology and anesthesia services to evaluate patients with pheochromocytoma or who are otherwise at risk for hypertensive crisis during adrenal tumor ablation prior to intended ablation. Preprocedural assessment, intraprocedural monitoring, and postablation evaluation of hemodynamics are critical to safe and successful ablation in this setting. Moreover, it is essential that a patient-specific medical regimen for the mitigation of hypertensive crisis is developed and executed. This plan must take into account an individual patient’s medical history and preprocedural, intraprocedural, and postablation hemodynamics to ensure safe, optimal medical management. aGeneral anesthesia with intra-arterial blood pressure monitoring should be considered prior to thermal ablation of these tumors. | |||||
Depending on the proximity of the target tumor to vulnerable regional anatomy, hydrodissection may be warranted prior to thermal ablation to ensure safe and successful therapy. It is most useful in protecting the bowel and pancreas and may also facilitate protection of the lung, kidney, or hemidiaphragm and chest wall (including intercostal nerves). 14 Under sonographic guidance a 20-to 22-gauge needle is used to instill a small (~ 50 mL) volume of 5% dextrose in water (D5W) into the targeted location. Ionic solutions like normal saline can conduct electricity, and should not be used for hydrodissection prior to RFA, although they may be used for cryoablation procedures. 14 After confirming initial needle placement and location of the hydrodissection fluid, a needle-sheath catheter is typically inserted into the initially instilled fluid volume (e.g., Yueh centesis catheter, Cook Medical; or Skater centesis catheter, Angiotech). Its needle stylet is then removed, and additional fluid is hung to gravity with a stopcock in series. 14 Fluid should flow freely if in a peritoneal or retroperitoneal location, with the location and distribution of the fluid and its displacement of regional anatomy assessed with serial CT. Iodinated contrast media (10 mL) may be added to the fluid to improve visualization of the instilled fluid under CT guidance. The total volume of fluid instilled depends on the amount of organ displacement needed to avoid thermal injury to nontarget tissue. 14 If initial attempts at hydrodissection do not adequately protect or displace the anatomical structure of concern, patient repositioning and additional instillation of fluid may be required, as well as probe repositioning, to ensure a safe and optimal relationship between the ablation probe, tumor, and nontarget anatomy. 14
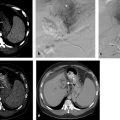
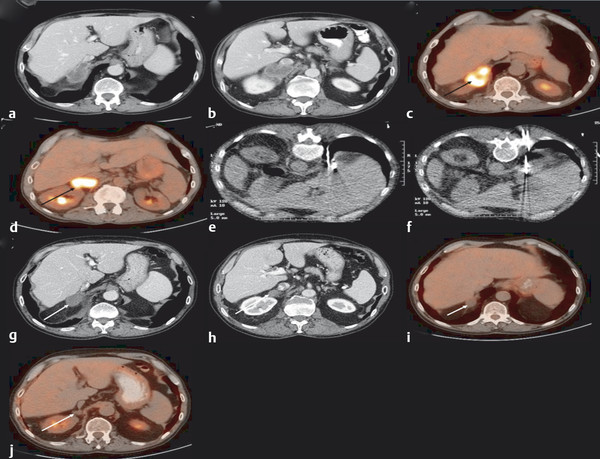
Cystic tumor elements can add to procedural complexity. When treating a cystic or mixed cystic and solid adrenal tumor with RFA, the RFA probe may need to be moved multiple times to ablate the solid components and ensure complete treatment. 14 In such cases, MWA may be used to provide optimal heating. 14 , 26 Longer treatment times may be required for cystic lesions, and coagulation may be sudden when it eventually occurs.( Fig. 2.3 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree