8 Colorectal Cancer Hepatic Metastatic Disease: Intra-arterial Therapies
8.1 Introduction
Colon cancer is the second leading cause of death from cancer in the United States. 1 The prognosis of colorectal carcinoma primarily depends on the presence of distant metastases. 2 , 3 There were an estimated 142,820 new cases of colon cancer and 50,830 deaths attributed to the disease in the United States in 2013. 4 Liver-only metastasis affects approximately 50% of patients. 1 Liver is the most common site, and about 40% of patients die with the liver as their only site of metastasis. 5 Hepatic metastases are initially resectable in about 25% of cases, and resection of liver metastases has been shown to improve long-term survival. 1 , 6
For patients with nonresectable liver metastasis, systemic chemotherapy remains the standard first-line therapy. However, despite significant improvements in modern systemic regimens containing both conventional and molecular targeted agents, most patients eventually develop progressive disease within months. 7 , 8 Over the last 2 decades, several intra-arterial locoregional therapies for the treatment of liver-only or liver-dominant hepatic colorectal cancer (hmCRC) have been developed. These therapies take advantage of the fact that the tumor vascularity is derived nearly 100% from the hepatic artery, whereas normal liver parenchyma derives only 30% of its blood supply from the hepatic artery and 70% from the portal vein. This chapter covers the intra-arterial treatment options for hmCRC, including transarterial chemoembolization (TACE) using either emulsions of ethiodized oil and chemotherapy solution (conventional TACE), TACE using drug-eluting beads loaded with irinotecan (DEBIRI-TACE), and radioembolization with microspheres labeled with the β emitter yttrium-90 (90Y).
8.2 Chemoembolization
Chemoembolization combines transarterial local delivery of high-dose chemotherapy and local ischemia by means of embolization. The embolic particles slow the passage of chemotherapy agents through the hepatic circulation, and the drug concentrations in the tumor can reach levels up to 25 times greater than levels reached with standard intravenous (IV) infusion. The drugs also remain within tumor cells for as long as 1 month after infusion, greatly augmenting the therapeutic effect. 9 , 10 , 11 , 12 Embolization also causes ischemia, resulting in tumor hypoxia, which has been shown to augment the effects of cytotoxic drugs by increasing their uptake and retention by tumor cells. 13
8.2.1 Indications
Patients with unresectable or nonablatable liver-only or liver-dominant metastases, or those who cannot undergo surgery due to comorbidities, are potential candidates for both transarterial embolization techniques. Candidates should have a life expectancy of more than 3 months with an Eastern Cooperative Oncology Group (ECOG) status ≤ 2. Patients should also have sufficient functional liver reserve. Although there is no consensus on the optimal functional reserve of liver, a bilirubin level of > 2 mg/dL, an albumin level of < 3 g/dL, and an international normalized ratio of 1.6 or higher are considered indicators of insufficient liver functional reserve. 14
8.2.2 Contraindications
Patients with contraindications to angiography, such as anaphylactoid reaction to iodinated contrast or uncorrectable coagulopathy, are excluded from treatment. Patients who cannot receive chemotherapy due to severe thrombocytopenia (< 50,000), leucopenia (absolute neutrophil count < 1,000), severe renal insufficiency (creatinine > 2 mg/dL), or severe cardiac dysfunction (American Heart Association [AHA] class III–IV failure) are also excluded from therapy. Hepatic encephalopathy or other signs of liver failure are also considered contraindications.
Portal vein occlusion is a relative contraindication. However, these patients can be treated safely as long as there are sufficient collaterals with hepatopetal flow. 15 Biliary obstruction is another relative contraindication. Even with a normal bilirubin level, the presence of biliary ductal dilatation puts patients at risk for bile duct necrosis and biloma formation. The presence of a bilioenteric anastomosis, a biliary stent, or prior sphincterotomy causes colonization of the bile ducts with enteric bacteria. These patients are at high risk of developing liver abscess following treatment. Aggressive prophylactic antibiotic regimens can reduce the incidence of this complication. 16
8.2.3 Preprocedure Workup
Patients for TACE (whether conventional TACE or DEBIRI-TACE) should be evaluated in the interventional oncology clinic. A detailed oncological history should be obtained, including the tissue diagnosis, prognostic markers such as KRAS and BRAF, presence of synchronous versus metchronous metastasis, time interval from diagnosis of primary cancer to the appearance of metastasis and details of prior treatment (chemotherapy, surgery or radiation therapy). It is important to emphasize the palliative role of intra-arterial therapies during the clinic visit, and both the physician and the patient should agree on clear, realistic goals.
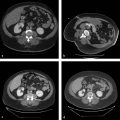
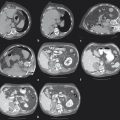
The imaging workup should include triple-phase computed tomography (CT) or magnetic resonance imaging (MRI), which needs to be evaluated for tumor burden, distribution, status of the portal vein and biliary tree, and presence and extent of extrahepatic disease. Patients with > 70% liver volume involvement with tumor are unlikely to benefit from TACE and should be discouraged from pursuing chemoembolization.
Laboratory evaluation should include complete blood count (CBC), coagulation studies, renal function tests, liver function tests, and carcinoembryonic antigen (CEA) level.
8.2.4 Conventional Transarterial Chemoembolization
At present, there is no standardized protocol for conventional TACE for the treatment of hmCRC. The chemotherapy agents used vary between centers. Studies comparing different chemotherapy regimens have not revealed a superior combination. 17 Combination therapy with cisplatin, doxorubicin, and mitomycin C is the most commonly used regimen in the United States, whereas monotherapy with doxorubicin is most frequently used worldwide. 18 All of these drugs have shown significantly higher target drug concentration when administered intra-arterially compared to intravenously. 19
Many agents are used to achieve embolization in conventional TACE. Pharmacokinetic data suggest that the chemotherapeutic drugs in the aqueous phase of the solution will wash out unless efflux is simultaneously blocked by the particles. 18 The materials used include polyvinyl alcohol, gelatin sponge, starch microspheres, and collagen particles. Most protocols include Ethiodol, or ethiodized oil, an iodinated ethyl ester of poppy seed oil (Guerbet). Some regimens involve delivery of the chemotherapeutic agents and oil emulsion followed by particulate embolization.
8.2.5 Technique
Patients are admitted to the interventional radiology service on the morning of the procedure following overnight fasting. Vigorous IV hydration is important. Premedication with prophylactic IV antibiotics and antiemetics is preferred, and both are continued until discharge. However, the use of antibiotic prophylaxis is not strictly evidence based. The procedure is performed under conscious sedation.
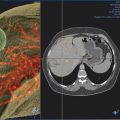
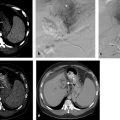
The procedure starts with detailed diagnostic visceral arteriography. 20 Power-injected digital subtraction angiograms of the superior mesenteric and celiac angiograms are obtained to identify variant anatomy, such as replaced or accessory hepatic arteries, nontarget branches to the viscera, hepatic branch anatomy, and patency of the portal vein. If nontarget branches are identified, these vessels must be either embolized using coils or avoided by placing the catheter tip well beyond their origin. Cone-beam CT is a valuable adjunct to identify target and nontarget tissue perfusion, and its routine use has been demonstrated to improve clinical outcomes in chemoembolization. 21 Complete mesenteric arteriography needs to be performed prior to only the first session. Subsequent chemoembolizations usually require detailed angiography of only the specific vessel(s) supplying the segments to be treated.
Once the arterial anatomy and tumor supply are clearly identified, a microcatheter is advanced superselectively into the right or left hepatic arterial branches. Whole-liver chemoembolization is not recommended due to unacceptable toxicity. 22 Segmental or subsegmental delivery of the chemoembolic agent is preferred when liver function is marginal. Once the catheter is positioned for treatment, a final arteriogram is performed to delineate the vascular territory prior to the chemotherapy injection.
The chemoembolic mixture or emulsion is injected in 1 to 5 mL increments until near stasis. Excessive embolization must be avoided, particularly for patients in whom repeated chemoembolizations are anticipated. After satisfactory treatment, the vessels should appear as a “tree in winter,” with no tumor blush, but with preservation of flow in the segmental and lobar branches. For bilobar disease, patients will require two to four treatments, depending on the arterial supply.
8.2.6 Side Effects and Complications
Postembolization syndrome occurs in approximately 67% of patients undergoing TACE, with patients complaining of varying degrees and combinations of abdominal pain, nausea, fever, fatigue, and elevated liver enzymes. 14 Because the hepatic arteries are the main supply for the biliary tree, bile duct injury occurs in 5.3 to 11.3% of cases. 23 , 24 Patients whose right hepatic artery is chemoembolized proximal to the cystic artery may experience a prolonged postembolization syndrome and may develop sterile ischemic chemical cholecystitis that resolves with conservative treatment. 25 The incidence of major complications after chemoembolization is 2 to 7%. 26 Major complications of hepatic embolization include hepatic insufficiency or infarction, abscess, biliary necrosis, and nontarget embolization of the gut. Other complications occur < 1% of the time, including periprocedural cardiac events, renal insufficiency, anemia requiring transfusion, and complications related to angiography. Thirty-day mortality rates have been reported to be 1 to 4%.
8.2.7 Outcomes of Conventional Transarterial Chemoembolization
Studies assessing conventional TACE for the treatment of hmCRC show significant heterogeneity in the patient population. Currently, conventional TACE is used as salvage therapy in patients with tumors refractory to other treatments, and patients selected for TACE often have more complex and extensive disease.
Three of the largest studies on survival data following conventional TACE for hmCRC permit subgroup analyses. Vogl et al 17 reported a 10-year series of 564 patients chemoembolized with either mitomycin alone (43%), mitomycin and gemcitabine (27%), mitomycin and irinotecan (15%), or mitomycin, irinotecan, and cisplatin (15%), depending on their prior systemic therapy, with Lipiodol and starch microspheres. All patients had progressed or become intolerant of systemic chemotherapy. Patients with liver involvement of > 70%, or ECOG performance status > 1 were excluded. The mean number of embolizations per patient was 6, with a range of 3 to 29. Partial response by Response Evaluation Criteria in Solid Tumors (RECIST) was seen in 17%, with disease control in 65%. Median survival from time of chemoembolization was 14.3 months, with no difference among the drug regimens. Eighty-four patients (15%) were downstaged to potentially curative resection or ablation, which was predictive of better survival. The presence of extrahepatic disease did not affect survival (median 13.8 mo vs. 12 mo, p = 0.68).
Vogl reported separately on a subset of 224 patients with up to five metastases with none > 5 cm, who were chemoembolized, followed 1 month later by thermal ablation with magnetic resonance (MR)-guided laser thermometry. 27 Only 2 of 464 ablated metastases developed local recurrence. The median time to progression of disease was 8 months, almost entirely due to the appearance of new metastases. Additional chemoembolizations and ablations were performed as indicated for recurrences. The median survival from initiation of chemoembolization was 23 months, with actuarial survival of 88% at 1 year, 49% at 2 years, and 19% at 5 years.
Albert et al reported a retrospective series of 121 patients chemoembolized with CAM (Cisplatin, Adriamycin and Mitomycin), Lipiodol, and polyvinyl alcohol (PVA). 28 The disease control rate was 43%, with a median survival of 2 months from diagnosis of metastases and 9 months from the time of chemoembolization. ECOG performance status > 0 and prior treatment with more than two lines of systemic therapy were negative prognostic factors, but the presence of extrahepatic disease was not.
8.3 DEBIRI-TACE
Drug–eluting beads allow for controlled release of a fixed dose of chemotherapy agents that are loaded onto microspheres. They achieve simultaneous embolization and delivery of chemotherapeutic agent to the target tumor. Irinotecan is the most common drug that is loaded onto the microspheres. Irinotecan is a camptothecin derivative that inhibits the production of the enzyme topoisomerase I, which is essential to DNA replication in cancer cells. Irinotecan is used as a second-line treatment for advanced colorectal cancer as part of FOLFIRI (5-fluorouracil [5-FU], leucovorin, and irinotecan) (FOLFIRI) or as a single agent in patients who have failed an established 5-FU-containing treatment regimen.
DC Beads (Biocompatibles), known as LC Beads in the United States, is the most extensively studied drug-eluting embolic. It is a soft, deformable material composed of PVA hydrogel with sulfonate groups. Positively charged drugs like irinotecan interact with the anionic charge in the sulfonate group by an ion-exchange mechanism. The elution of the drug from the beads happens in the presence of counterions, such as sodium, potassium, or calcium (Na, K, or Ca) in the plasma. The beads are approved in Europe for embolization and loading with doxorubicin. In the United States, the addition of the chemotherapeutic agent is considered an off-label application. The size varies from 70 to 900 µm, and the spheres are stored in a phosphate packaging solution.
8.3.1 Chemotherapy Loading
Loading is done in the pharmacy under aseptic conditions. Beads come in 10 mL sterile vials containing 2 mL of sedimented beads in phosphate-buffered saline. The saline is removed from the vial and irinotecan is loaded from 5 mL vials of Campto (Pfizer Inc.) containing 100 mg of irinotecan hydrochloride in liquid form. Loading time is variable, depending on the size of the beads. Average loading time is 2 hours. Smaller beads need shorter loading times due to the greater surface area. 29 During the process, the beads must be shaken for effective loading. If the beads have been correctly loaded with irinotecan, the color changes to turquoise. At the end of the loading time, excess solution must be removed from the vial and discarded.
The loaded beads can be stored up to 14 days under refrigerated conditions (2–8°C). The loaded beads are mixed with contrast during the procedure and are used immediately because some drug elution is initiated in the process. Because the drug release is driven by ion exchange, nonionic contrast should be used. 30 Saline is not recommended for preparing suspensions of loaded beads. Prior to usage, any supernatant containing irinotecan should be removed from the vial of before mixing with 5 mL of nonionic contrast medium and 5 mL of sterile water. The syringe is then gently inverted to obtain an even suspension of beads. For a standard procedure, 100 to 300 μm DC beads are recommended. Each vial contains 2 mL of beads and is loaded with 100 mg irinotecan (loading dose, 50 mg irinotecan/mL of beads).
8.4 Peri- and Intraprocedural Management
Compared to conventional TACE, DEBIRI-TACE is associated with a higher incidence of pain and other adverse effects, such as nausea and vomiting. Patients are typically admitted the night before the procedure, and IV hydration is started with normal saline at the rate of 100 mL/h. Esomeprazole 40 mg is administered intravenously on the day of admission, 30 minutes prior to the procedure, and a second dose is given the day after the procedure. Palonosetron 0.25 mg is started intravenously 30 minutes prior to the procedure. IV morphine 10 mg is administered prior to injection of the beads and is followed by a second dose 6 hours after the procedure. Other medications include IV dexamethasone 20 mg 30 minutes prior to chemotherapy, ondansetron 8 mg IV 30 minutes prior to chemotherapy, and 8 mg IV 6 hours postchemoembolization. Antibiotic coverage is with cefazolin 1 gram intravenously IVPB 6 hours prior to chemotherapy, and Flagyl (Pfizer) 500 mg IVPB every 8 hours, continued while the patient is admitted. The medications are listed in Table 8.1 .
Day 0 (day before TACE) | Day 1 (day of TACE) | Day 2 (day after TACE) |
Esomeprazole 40 mg, IV | Esomeprazole 40 mg, IV (30 minutes prior to TACE) | Esomeprazole 40 mg, IV |
Intravenous hydration with normal saline @100 mL/h | Intravenous hydration with normal saline @100 mL/h | Intravenous hydration with normal saline @100 mL/h (until adequate oral intake) |
Ondansetron 8 mg, IV (30 minutes prior to TACE and 6 hours after the procedure) | Ondansetron 8 mg, IV, every 6 hours, as needed | |
Metronidazole 500 mg, IV every 8 hours | Metronidazole 500 mg, IV every 8 hours (until discharge) | |
Dilaudid PCA postprocedure | Dilaudid PCA postprocedure (as needed) | |
Morphine 10 mg, IV (prior to injection of beads and 6 hours after the procedure) | ||
Dexamethasone 20 mg, IV (30 minutes prior to TACE) | ||
Palonosetron 0.25 mg, IV (30 minutes prior to TACE) | ||
Cefazolin 1 g, IV (preprocedure prophylactic antibiotic) | ||
Abbreviations: DEBIRI, drug-eluting beads with irinotecan; IV, intravenous; PCA, patient-controlled analgesia; TACE, transarterial chemoembolization. | ||
8.4.1 Technique
Initial catheterization and angiographic techniques for DEBIRI-TACE are the same as already described for conventional TACE. Embolization is started after the microcatheter is positioned in the target vessel. An injection rate of approximately 1 mL/min of the beads–contrast suspension is recommended. Injection of intra-arterial lidocaine (4–10 mL split before and near the end of DEBIRI administration) has been shown to reduce the postprocedure pain and length of hospital stay. 31 It is important to realize that the goal of transcatheter treatment with DEBIRI is to deliver the planned dose of anticancer agent, not to occlude the vessel. In a multi-institutional registry, achievement of complete stasis was an independent predictor of adverse events and significantly greater hospital length of stay. 31
It is important to maintain forward flow into the vessel throughout the procedure. If “near stasis” is observed during the injection (i.e., the contrast column does not clear within 2–5 heartbeats) before the full planned dose has been administered, the injection should be stopped at that time, regardless of the amount of beads that have been actually delivered, to avoid reflux of embolic material. Additional embolic material of any kind should not be injected following the delivery of DEBIRI, even if the full dose has been delivered with maintained forward flow.
Transarterial delivery of the beads is performed in a lobar fashion. In patients with unilobar disease, two lobar treatments are planned, each with 100 mg irinotecan loaded in one vial of 100 to 300 µ (micron) DC Beads. Repeat treatment is separated by 3 to 4 weeks after ensuring that the liver enzymes have returned to baseline. In patients with bilobar disease four lobar treatments should be planned, each with 100 mg irinotecan loaded in one DC Bead vial separated by a minimum interval of 2 weeks.
8.4.2 Outcomes of DEBIRI-TACE
Many prospective trials have shown that DEBIRI-TACE is safe and effective in hmCRC. Fiorentini reported a prospective, multi-institutional, double-arm study of 74 patients randomized to receive DEBIRI (n = 36) or systemic chemotherapy (FOLFIRI) (n = 38). 32 Overall response rate (Complete response [CR] + Partial response [PR]) in the liver in the DEBIRI group was 68.6% (n = 24) compared with 20% (n = 7) in the systemic treatment group. Median survival was 22 months for DEBIRI and 15 months for FOLFIRI. At 50 months, overall survival was significantly longer for patients treated with DEBIRI than for those treated with FOLFIRI. Progression-free survival was 7 months in the DEBIRI group compared to 4 months in the FOLFIRI group.
Martin et al reported a prospective, multi-institutional, single-arm study of 55 patients treated with DEBIRI. Ninety-nine DEBIRI treatments were performed, with a median of 2 (range 1–5) per patient. 33 Response rates were 66% at 6 months and 75% at 12 months. Overall median progression-free survival (PFS) was 11 months with a median hepatic-specific PFS of 15 months and a median overall survival of 19 months.
Richardson et al reported a comprehensive review of five observational studies and one randomized, controlled trial (RCT) describing the use of DEBIRI in the treatment of hmCRC (total of 235 patients). 34 The median survival time in this systemic review was 15 to 25 months. There was an improvement in disease-free survival associated with DEBIRI. The response rate (CR+PR) varied from 36 to 78%. Patients with response at 6 months showed a durable response up to 12 months.
Narayanan et al reported a retrospective study of 28 patients treated with 47 DEBIRI-TACE procedures. Three patients (15%) had a complete response, six (30%) had a partial response, four (20%) had stable disease, and disease progression was recorded in seven (35%); CT scans were unavailable for eight patients. 35 The median time from diagnosis of liver metastases to initial DEBIRI treatment was 19.6 months. The median overall survival from first treatment was 13.3 months.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree