12 Portal Vein Embolization
12.1 Introduction
Liver transplantation, surgical resection, and percutaneous ablative approaches are potentially curative treatments for patients with hepatic malignancies. Transplantation criteria have been rigorously designed given the scarcity of available donor livers to ensure patients with the most critical need receive transplants; thus surgical resection and percutaneous ablation are the main curative therapies for liver malignancies. Patients with underlying liver disease or large tumor burden may develop perioperative hepatic failure following hepatic resection. Portal vein embolization (PVE) is a procedure that takes advantage of the regenerative properties of the liver by redirecting portal venous blood flow from liver segments to be resected to the future liver remnant (FLR) for induction of hepatic hypertrophy. PVE has been shown to improve the postoperative outcomes of patients following hepatic resection, and therefore has become an established standard of care prior to major hepatectomies. 1 , 2
12.2 Indications
Portal vein embolization is indicated when the future liver remnant is deemed insufficient to provide adequate hepatic function following hepatic resection. 3
12.3 Contraindications
One absolute contraindication to PVE is the presence of extensive portal venous tumor thrombi. Large tumor thrombi divert portal venous blood to the future liver remnant, and attempts to perform additional PVE can result in deleterious nontarget embolization of the FLR. 4
A second absolute contraindication to PVE is clinically evident portal hypertension, which is considered a contraindication to hepatic resection. However, many investigators have demonstrated similar postoperative course and survival benefits following hepatic resection in patients with and without portal hypertension; thus hepatectomy in this group of patients remains a topic of debate. 4 , 5 , 6 , 7
Relative contraindications to PVE are uncorrectable coagulopathy, renal insufficiency, intrahepatic biliary dilation in the FLR, and extrahepatic metastatic disease.
12.4 Patient Selection and Preprocedural Workup
The formation of a multidisciplinary team of hepatologists, medical oncologists, surgical oncologists, radiologists, and interventional radiologists is of immense importance in the selection of appropriate patients for PVE and subsequent hepatic resection.
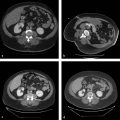
The preprocedural workup includes evaluating the patient for baseline liver function, acquiring volumetric cross-sectional examination of the liver, and determining the extent of liver resection. Traditionally, computed tomographic (CT) examinations of the liver have been used for assessment of FLR and estimated total liver volume. However, magnetic resonance imaging (MRI) examination of the liver can also be used to compute hepatic volumes ( Fig. 12.1 ).
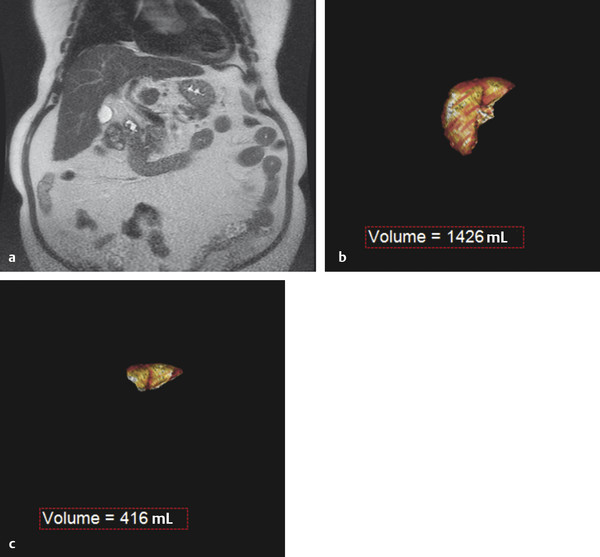
Computer software designed for manipulation of volumetric data can permit the calculation of FLR volume and the total liver volume. The FLR volume is calculated by tracing the FLR on each axial image. The total estimated liver volume (TELV) can be computed by tracing the entire liver border on each axial image, with or without subtracting the outline of the tumor(s). Alternatively, the total liver volume can be estimated using the following formula: Total Estimated Liver Volume = − 791.41 + 1,267.28 × Body Surface Area. 8 A standardized FLR (sFLR) is the ratio of the FLR volume to the TELV expressed as a percentage.
In patients with normal hepatic function, an sFLR of > 20% is associated with reduced length of hospital stay and reduced postoperative complications following hepatic resection. 9 , 10 Accordingly, PVE is recommended for patients with normal hepatic function and an sFLR of < 20%. 11
In patients with hepatotoxic liver injury resulting from chemotherapy, PVE is recommended when the sFLR is < 30%. 10 , 12 Similar to systemic hepatotoxic chemotherapy, hepatic steatosis is typically a diffuse hepatic parenchymal process resulting in hepatic injury and dysfunction; therefore, PVE is recommended when the sFLR is also < 30%.
Patients with chronic liver disease, such as cirrhosis and retained hepatic function, with good functional status can be considered for hepatic resection. PVE is recommended for cirrhotic patients when the sFLR is < 40%. 10 , 12
12.5 Technique
The procedure is usually performed under conscious sedation and sometimes with general anesthesia. Portal venous access is established via an ipsilateral or contralateral percutaneous transhepatic approach. 4 , 13 , 14 , 15 The ipsilateral approach involves access into the hepatic lobe containing the tumor(s). Two advantages of the ipsilateral approach are avoidance of injury to the FLR with needle access, sheath placement, and catheter manipulations and ease of selection of segment 4 branches, assuming a right portal venous access for a planned extended right hepatectomy. The main disadvantage of the ipsilateral approach relates to the selection of the right portal veins, which can be difficult given the acute angulation of right portal veins with respect to the site of access.
The contralateral percutaneous transhepatic approach involves an initial access in the FLR. The main advantage of the contralateral approach is the ease in which right portal venous branches can be selected, and the shorter catheter lengths required, thus reducing the amount of catheter dead space. Limiting the catheter dead space is particularly important when using N-butyl cyanoacrylate (NBCA) and Lipiodol (Guerbet) mixture for embolization. The main disadvantage of the contralateral approach is potential for injury to the FLR. The ipsilateral and contralateral techniques described here specifically discuss the use of particles and coils for PVE; however, other embolic agents may also be employed.
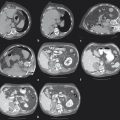
The ipsilateral approach involves access into a right-sided peripheral portal venous branch using a 21- or 22-gauge Chiba needle ( Fig. 12.2 ). This may be performed under fluoroscopy using landmarks, or under direct ultrasound guidance. The access is usually along the right midaxillary line. Appropriate needle access into the peripheral portal venous branch can be confirmed with injection of contrast through the needle. At this time, a 0.018 in wire is inserted into the needle and advanced under fluoroscopic guidance into the main portal vein. Applying the Seldinger technique, a 5 or 6 French vascular sheath is advanced into the right portal vein to permit introduction of catheters. Initially, an anteroposterior flush portal venogram using a 5 French flush catheter is performed to delineate the portal venous anatomy. Rotational angiography with three-dimensional (3D) reconstruction of the portal venous system can also be performed as a convenient reference during the procedure.
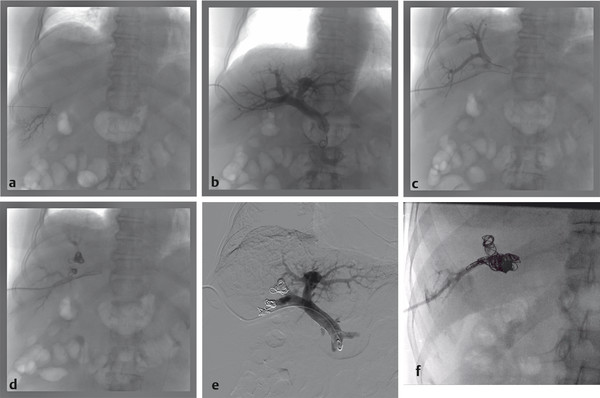
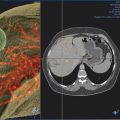
When embolization involves segment 4, some authors advocate segment 4 embolization first given the potential difficulty of catheter exchanges and possible dislodgment of embolic agents following embolization of the right-sided portal venous system. 10 , 12 Segment 4 can be selected using a 0.035 in hydrophilic guidewire (Glidewire, Terumo Medical Corp.) and several 5 French catheters (i.e., Kumpe catheter, Cook Medical; Glidecath, Terumo Medical Corp.). Then a microcatheter (i.e., 3 French Renegade Hi-Flo catheter, Boston Scientific; 2.8 French Progreat catheter, Terumo Medical Corp.) is advanced coaxially through the 5 French catheter more distally into segment 4 branch for delivery of embolic agents ( Fig. 12.3 ). Some authors embolize the portal veins with particles of increasing size, ranging from 100 to 700 µm in diameter, until stasis, followed by coil embolization of the proximal segment 4 vein to further reinforce portal venous flow diversion. Attention is then directed to the rest of the right portal venous branches. The 5 French catheter is exchanged for a 5 French reverse-curve catheter to select the right portal venous branches (Simmons 1, Simmons 2, Cook Medical, Bloomington, IL. Sos Omni 2, AngioDynamics). Through the reverse-curve catheter, a microcatheter is advanced more distally where particle embolization is performed until stasis, followed by coil embolization. Finally, the reverse-curve catheter is exchanged for a flush catheter for a flush portal venogram to confirm occlusion of the embolized branches. The catheter and sheath are subsequently removed, and the access tract is occluded with Gelfoam or coils.
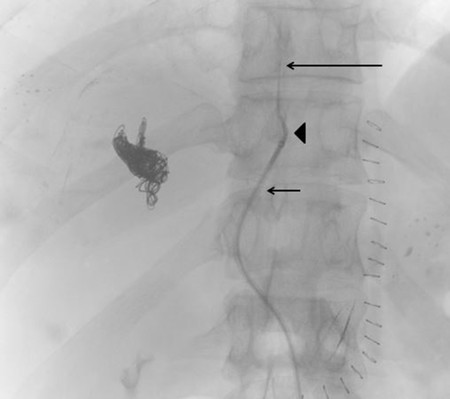
The contralateral percutaneous transhepatic approach involves an initial access in the FLR. Access is achieved into a peripheral branch of the left hepatic lobe, usually segment 3, using a 21- or 22-gauge Chiba needle under ultrasound guidance. Selection of the right and segment 4 portal venous branches can be achieved using a standard 5 French catheter (Kumpe catheter, Cook Medical) and a coaxially inserted microcatheter (Renegade Hi-Flo, Boston Scientific) without an associated sheath.
There are many embolic agents and combinations of embolic agents that have been employed for PVE, including gelatin sponge, fibrin glue, NBCA glue, thrombin, polyvinyl alcohol particles, Amplatzer vascular plugs (St. Jude Medical), embolization coils, and Lipiodol. As mentioned previously, the techniques for PVE are the same regardless of the embolic agent used. It is worth stating that some studies report that the use of NBCA results in a greater volume of FLR increase. 16 , 17
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree