The incidence of haematological neoplasms
Cancer is an increasingly important cause of morbidity and mortality with recent improvements in the prevention and treatment of cardiovascular disease. Nearly 40% of the population will develop cancer in their lifetime. The majority of cancers are epithelial malignancies. Haematological malignancies represent approximately 7% of all malignant disease (Fig. 11.2). There are major geographical variations in occurrence of the diseases; for example, chronic lymphocytic leukaemia (CLL) is the most common leukaemia in the West but rare in the Far East.
Figure 11.2 The relative frequency of the haematological malignancies as a proportion of malignant disease. (From Smith A. et al. (2009) Br J Haematol148, 739–53, with permission.)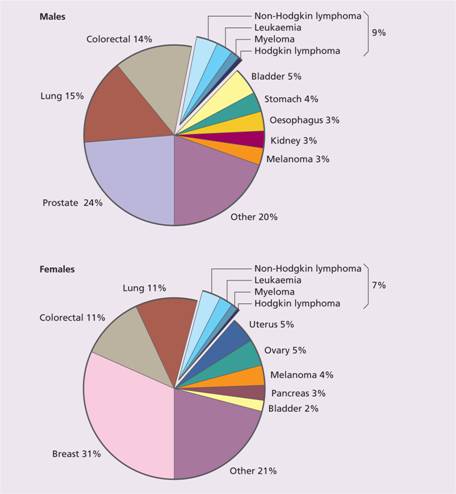
The aetiology of haemopoietic malignancy
Exactly how genetic mutations accumulate in haemopoietic malignancies is largely unknown. As in most diseases it is the combination of genetic background and environmental influence that determines the risk of developing a malignancy. For example, single nucleotide polymorphism (SNP) analysis has identified polymorphisms in germline genes, some of which code for proteins involved in B-cell development, that predispose to risk of B-cell acute lymphoblastic leukaemia (B-ALL) (see Chapter 17). However, in the majority of cases neither a genetic susceptibility nor an environmental agent is apparent.
Inherited factors
The incidence of leukaemia is greatly increased in some genetic diseases, particularly Down’s syndrome (where acute leukaemia occurs with a 20- to 30-fold increased frequency), Bloom’s syndrome, Fanconi’s anaemia, ataxia telangiectasia, neurofibromatosis, Klinefelter’s syndrome and Wiskott–Aldrich syndrome. There is also a weak familial tendency in diseases such as acute myeloid leukaemia (AML), CLL, Hodgkin lymphoma and non-Hodgkin lymphoma (NHL) although the genes predisposing to this increased risk are largely unknown.
Environmental influences
Chemicals
Chronic exposure to benzene is an unusual cause of myelodysplasia or AML. Other industrial solvents and chemicals less commonly cause leukaemia.
Drugs
The alkylating agents (e.g. chlorambucil, melphalan, procarbazine and nitrosoureas – BCNU, CCNU) predispose to AML, especially if combined with radiotherapy. Etoposide is associated with a risk of the development of secondary leukaemias associated with balanced translocations including that of the MLL gene at 11q23.
Radiation
Radiation, especially to the marrow, is leukaemogenic. This is illustrated by an increased incidence of all types of leukaemia (except CLL) in survivors of the atom bomb explosions in Japan.
Infection
Children may have a predisposition to acute lymphoblastic leukaemia (ALL) from their germline constitution. A proportion of cases of childhood ALL are then initiated by genetic mutations that occur during development in utero (Fig. 11.3). Studies in identical twins have shown that both may be born with the same chromosomal abnormality, e.g. t (12; 21). This has presumably arisen spontaneously in a progenitor cell that has passed from one twin to the other as a result of the shared placental circulation. Environmental exposure during pregnancy may be important for this first event. One twin may develop ALL early (e.g. at age 4) because of a second transforming event affecting the copy numbers of several genes including those in B – cell development and relevant to the leukaemogenesis. The other twin remains well or may develop ALL later. The TEL–AML1 translocation is present in the blood of approximately 10% of newborn infants but only 1 in 100 of these go on to develop ALL at a later date. The mechanism of the ‘second genetic hit’ within the tumour cell is unclear but an abnormal response of the immune system to infection is suggested by epidemiological studies. Children with a high level of social activity, notably those attending early nursery daycare, have a reduced incidence of ALL, whereas those living in more isolated communities and who have a reduced exposure to common infections in the first years of life have a higher risk.
Figure 11.3 Prenatal origin of acute lymphoblastic leukaemia (ALL) in a pair of identical twins. ALL was diagnosed in the first twin at age 5 years and in the second at age 14 years. Both tumours had an identical t (12; 21) translocation indicating probable origin of the leukaemic clone in utero and dissemination to both twins via a shared placental blood supply. Because of the prolonged latency of the ALL it is presumed that a secondary event is required to initiate the development of frank leukaemia. At the time of the diagnosis of ALL in twin 1 the t (12; 21) translocation could be detected in the bone marrow of twin 2. It is likely that such a ‘fetal origin’ of childhood ALL occurs in a significant number of sporadic ALL cases. (After Wiemels J.L. et al. (1999) Blood94, 1057–62.)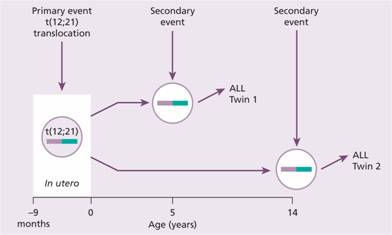
Viruses
Viral infection is associated with several types of haemopoietic malignancy, especially different subtypes of lymphoma (see Table 20.2). The retrovirus human T-lymphotropic virus type 1 is the cause of adult T-cell leukaemia/lymphoma (see p. 243) although most people infected with this virus do not develop the tumour. Epstein–Barr virus (EBV) is associated with almost all cases of endemic (African) Burkitt lymphoma, post-transplant lymphoproliferative disease (which develops during immunosuppressive therapy after solid organ transplantation (see p. 312)) and a proportion of patients with Hodgkin lymphoma. Human herpes virus 8 (Kaposi’s sarcoma-associated virus) is associated with Kaposi’s sarcoma and primary effusion lymphoma (see Table 20.2).
HIV infection is associated with an increased incidence of lymphomas at unusual sites such as the central nervous system. The HIV-associated lymphomas are usually of B-cell origin and of high-grade histology.
Bacteria
Helicobacter pylori infection has been implicated in the pathogenesis of gastric mucosa B-cell (MALT) lymphoma (see p. 265).
Protozoa
Endemic Burkitt lymphoma occurs in the tropics, particularly in malarial areas. It is thought that malaria may alter host immunity and predispose to tumour formation as a result of EBV infection.
The genetics of haemopoietic malignancy
Malignant transformation occurs as a result of the accumulation of genetic mutations in cellular genes. The genes that are involved in the development of cancer can be divided broadly into two groups: oncogenes and tumour-suppressor genes.
Oncogenes
Oncogenes arise because of gain-of-function mutations in normal cellular genes called proto-oncogenes (Fig. 11.4). Proto-oncogenes are involved in a variety of important cellular processes, often in the pathway by which external signals are transduced to the cell nucleus to activate genes. Oncogenic versions are generated when the activity of proto – oncogenes is increased or they acquire a novel function. This can occur in a number of ways including translocation, mutation or duplication. One of the striking features of haematological malignancies (in contrast to most solid tumours) is their high frequency of chromosomal translocations. A subset of proto-oncogenes are involved in control of apoptosis (e.g. BCL-2 which is overexpressed in follicular lymphoma) (see p. 266).
Figure 11.4 Proliferation of normal cells depends on a balance between the action of proto-oncogenes and tumour-suppressor genes. In a malignant cell this balance is disturbed leading to uncontrolled cell division.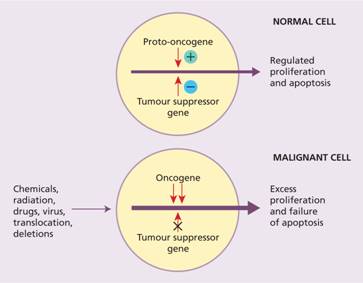
Tyrosine kinases
These enzymes, which phosphorylate proteins on tyrosine residues, are important as cell receptors and intracellular signalling. Mutations of them underlie a large number of haematological malignancies (see Chapters 13, 14 and 15).
Tumour-suppressor genes
Tumour-suppressor genes may acquire loss-of-function mutations, usually by point mutation or deletion, which lead to malignant transformation (Fig. 11.4). Tumour-suppressor genes commonly act as components of control mechanisms that regulate entry of the cell from the G1 phase of the cell cycle into the S phase or passage through the S phase to G2 and mitosis (see Fig. 1.8). Examples of oncogenes and tumour-suppressor genes involved in haemopoietic malignancies are shown in Table 11.1. The most significant tumour-suppressor gene in human cancer is p53 which is mutated or inactivated in over 50% of cases of malignant disease, including many haemopoietic tumours.
Table 11.1 Some of the more frequent genetic abnormalities within haematological tumours affecting the function of oncogenes.
| Disease | Genetic abnormality | Oncogenes involved |
| AML | t (8; 21) | ETO/AML1 (CBFα) |
| t (15; 17) | PML, RARA | |
| Nucleotide insertion | NPM | |
| Mutation, ITD | FLT3 | |
| Mutation | TET-2 | |
| Secondary AML | 11q 23 translocations | MLL |
| Myelodysplasia | – 5, del (5q) | RPS 14 |
| – 7, del (7q) | N RAS | |
| CML | t (9; 22) | BCR-ABL1 |
| Myeloproliferative | Point mutation | JAK-2 |
| Point mutation | TET-2 | |
| B-ALL | t (12; 21) | TEL/AML1 |
| t (9; 22) | BCR-ABL1 | |
| t (4; 11) | AF4/MLL | |
| Follicular lymphoma | t (14; 18) | BCL2 |
| Mantle cell lymphoma | t (11; 14) | Cyclin D1 |
| Burkitt lymphoma | t (8; 14) | MYC |
| CLL | 17p deletion | P53 |
| 11q 22-23 deletion | ATM |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


