11 Bone Tumors: Ablation
11.1 Introduction
Approximately 70% of the 1 million people that die each year in the United States due to cancer have breast, lung, or prostate cancer, and approximately one-half of these, or 350,000 people, will die with bone metastases. 1 Although bone metastases can indicate a poor prognosis with a median survival of 3 years or less, many patients now live many years with metastatic cancer, with 5 to 40% of patients alive at 5 years, dependent on tumor histology and burden. 2 , 3 Quality of life is an important consideration for patients, and unfortunately bone-related cancer pain is often undertreated, with nearly 80% of patients experiencing severe pain before a sufficient palliative treatment plan is initiated. 4
A multidisciplinary team that can offer optimal analgesic therapy, radiation therapy (RT), surgery, hormonal and chemotherapies, and focal image-guided ablation therapies provides the most effective management of patients with painful skeletal metastases. The front-line therapy for treatment of painful metastatic skeletal disease is external beam radiation therapy (EBRT). This treatment is at least partially effective for 50 to 80% of patients, with complete pain response in 50 to 60%. 5 Although many patients experience complete or partial relief of pain following RT, the time to pain relief can be extended, with the median relief of pain achieved in 3 to 7 weeks. 6 Although RT results in an initial reduction in pain for the majority of patients, at least for a period of weeks, 20 to 30% of patients do not experience pain relief, and a majority that had complete or partial response experience pain recurrence. 7 , 8 , 9 , 10 , 11 , 12 Retreatment is possible for many patients, but for many patients that experience minimal or transient relief of pain following EBRT, further treatment is not offered due to lack of response or due to limitations in normal tissue tolerance.
Surgery is generally reserved for lesions at great risk for fracture or following fracture, or for spinal metastases causing neurological compromise. Systemic therapies, including chemotherapy, hormonal therapy, radiopharmaceuticals, and bisphosphonates, in combination with opioid and nonsteroidal analgesics, can be effective treatment for widespread skeletal metastases. However, these are not generally offered for focal painful metastases because pain due to metastatic skeletal disease is often refractory. Radiopharmaceuticals are often effective in patients with diffuse painful bony metastases, but are not considered standard of care for patients with isolated, painful lesions. For many patients with focal painful metastatic disease that have failed EBRT, analgesics remain the only alternative treatment option that is typically offered. Many patients will limit their use of these medications due to significant side effects, such as constipation, nausea, and sedation.
Increasingly minimally invasive, percutaneous thermal ablation techniques have found clinical effectiveness for palliation for patients with limited skeletal metastases. These treatments use image guidance to place ablative devices into focal metastatic tumors. These ablation methods include the use of radiofrequency ablation (RFA), cryoablation, laser ablation, microwave ablation, and magnetic resonance–guided focused ultrasound (MRgFUS). In addition, patients at risk for fracture due to metastases in axially loaded locations (e.g., vertebral bodies and the periacetabular region) may benefit from percutaneous cementoplasty. Of these minimally invasive methods, RFA and cryoablation have been the most studied.
More recently, percutaneous ablation has been used to achieve local tumor control in patients with limited metastatic disease involving the musculoskeletal system. These patients with oligometastatic disease benefit from cost-effective minimally invasive treatments while avoiding the morbidity of surgery and, if new disease presents, retreatment is possible. 13 , 14 , 15
11.2 Indications
Patients that benefit from focal ablative therapy for painful metastatic disease report moderate or severe pain, typically ≥ 4 of 10 for the worst pain in a 24-hour period. Patients with lower reported pain scores are not usually treated because it is difficult to improve on mild pain, and this type of pain can usually be managed by oral analgesics. An important consideration is that pain should be limited to one or two sites, and the pain should correlate with a corresponding abnormality evident with cross-sectional imaging. For patients with multiple painful tumors or otherwise diffuse disease, identifying critical sites for treatment can be difficult, and it is also not practical to treat more than two or three sites in a typical treatment session. Often, multiple painful tumors are better treated with a systemic rather than focal approach. Tumors that are well suited to ablative therapy are most typically osteolytic or mixed osteolytic/osteoblastic in nature or otherwise composed of soft tissue. Osteoblastic lesions can also be treated, and this is commonly encountered in limited breast or prostate cancer. Treatment of osteoblastic disease requires the use of bone access devices or drills for access to the target tumor, and the ablation system used should be able to penetrate the sclerotic bone effectively. Adjacent critical structures, such as motor nerves or bowel, should be displaced using fluid or other displacement maneuvers to avoid collateral injury. Typically, a 1 cm margin between the target tumor and nearest critical structure is preferred, although operator experience and neural monitoring can allow safe treatment of tumors in closer proximity to these normal tissues. Patients at risk for fracture or risk for progression of fracture due to skeletal metastatic disease should be considered for surgery. If the tumor in question is in an axially loading location, augmentation with cement following ablation may also be helpful.
11.3 Contraindications
The most common technical contraindication for treatment of metastases involving bone or soft tissue is inaccessibility of the target tumor from a percutaneous approach. Close proximity of adjacent normal critical structures may also preclude ablation treatment. Safe treatment of tumors near normal critical structures can be achieved, although careful device placement, tissue displacement or other protective maneuvers, neural monitoring, and careful ablation monitoring are necessary. Absolute contraindications include uncorrectable bleeding diatheses and patient incompatibility with the level of anesthesia required to perform the procedure. Active infection is a strong relative contraindication, given the potential to seed necrotic, ablated tissue with circulating micro-organisms. Relative contraindications include widespread skeletal metastases, for which a systemic approach would be more appropriate, and mildly painful metastases, which are more suitable for analgesic medication and inconsistently respond to ablation.
11.4 Patient Selection and Preprocedural Workup
Patient history and preprocedural imaging are important to characterize the target tumors and to correlate with a patient’s symptoms. Imaging acquired at the time of presentation allows deliberate consideration of the potential risks versus the benefits of ablation. This imaging is also useful for planning adjunctive maneuvers or additional monitoring that may be of benefit during the procedure. The type of preprocedural imaging employed is dependent on the extent and site of disease. For patients that present with focal painful metastatic disease, computed tomography (CT) is often the modality of choice to determine technical feasibility. Because CT is frequently used to guide and monitor the ablation procedure, it is useful to demonstrate the target tumor and adjacent structures in treatment planning. Patients with disease involving the spine often benefit from the use of magnetic resonance imaging (MRI) to determine the full extent of disease, such as volume of disease within a vertebral body, involvement of adjacent vertebral bodies, and the presence of epidural or perineural involvement that may not be evident with CT imaging. MRI is also helpful in treatment of patients with oligometastatic disease because MRI more closely approximates the clinical, rather than the gross, extent of tumor burden. 16 Positron emission tomography (PET) with CT (PET-CT) offers the added benefit of demonstrating metabolic activity, which can add value for targeting tumors that have ill-defined borders on CT or previously treated/irradiated tumors that show surrounding bony changes (sclerosis or lucency) related to treatment effect rather than tumor infiltration. PET-CT is also helpful for staging patients with oligometastatic disease, and treatment goals are impacted by unexpected metastatic disease not previously identified.
11.5 Technique
11.5.1 Radiofrequency Ablation
RFA is the most commonly used percutaneous thermal tumor ablation method and also the most used method for treatment of painful metastatic disease involving bone. RFA can be performed with either general anesthesia or, with experience, moderate conscious sedation. A general anesthetic is more commonly used because the level of focal pain during the RFA treatment using monopolar systems can be greater than most patients can tolerate, even with moderate conscious sedation, and because the procedures can be long, lasting more than an hour depending on the size of the target lesion. More recently available bipolar systems used in treatment of metastatic skeletal disease do not cause pain during ablation, and moderate sedation is well tolerated. However, the use of general anesthesia allows the procedure to be performed without the additional necessity of providing supportive care for the patient as is required with conscious sedation. Less complex lesions (i.e., superficial, small, easily accessible, predominantly osteolytic or soft tissue lesions remote from normal vital structures) may be readily treated with patients under moderate sedation with sufficient local analgesia. Epidural spinal anesthesia or focal nerve blocks are often helpful to ease the pain during the immediate postablation period. If an epidural catheter is employed, the duration of use is typically for a 12- to 24-hour period following the RFA treatment using a monopolar system. Prior to removal of the catheter, it is helpful to halt the medication delivery for a trial period of several hours. If the patient’s pain has returned to the pretreatment level or is improved, the catheter can be removed. Most patients are observed overnight in the hospital to provide adequate pain control or to allow transition and modification of oral analgesic medication dosage. The patient is usually discharged with oral opioid analgesics for mild to moderate discomfort or pain.
For treatment of patients with tumors adjacent to important neural structures, intravenous conscious sedation permits intraprocedural focused neurological physical examination as a means of monitoring vulnerable neural structures. With the use of bipolar RFA systems, the patient can provide feedback when the ablation zone approaches neural structures because focal pain precedes nerve injury. Alternatively, use of intravenous anesthesia allows evoked potential monitoring for nerve monitoring with cases adjacent to motor nerves or the spinal cord. 16 Following ablation, instillation of long-acting local anesthetic along the periosteum may diminish postprocedural pain.
RFA procedures should be performed under appropriate cross-sectional imaging guidance and monitoring. Fluoroscopy may be useful for portions of the procedure, in particular for tumors contained in vertebral bodies. Fluoroscopy may also be used for treatment of patients with bipolar RFA systems as the end-point of the procedure is time and temperature patient pain, and thus not dependent on imaging. However, cross-sectional imaging is usually beneficial to carefully avoid critical structures and to estimate the treatment coverage for complex tumors. Ultrasound (US) may be used for superficial, predominantly soft tissue lesions, particularly in the chest wall or extremities. CT is the most commonly used modality for guidance, given its widespread availability for most practices and typical excellent delineation of the target tumor and surrounding structures. MRI possibly provides the best imaging for ablation monitoring with superior tumor depiction in bone; however, the MRI suite is a difficult environment for most procedures. Also, MRI-compatible devices remain limited, and when they are available, artifacts associated with devices can obscure the target tumor. Treatment of tumors involving bone or tumors adjacent to bone require percutaneous placement of RFA electrodes into the target tumor. RFA electrodes may be placed directly into soft tissue metastases or osteolytic skeletal metastases with destroyed or thin overlying cortex. To penetrate osteoblastic metastases or to access tumors deep to intact cortical bone, bone access devices may be required. These include manually operated bone biopsy needles or powered bone drills.
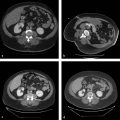
The targeted area of ablation includes the bone–tumor interface or the entire tumor, rather than simply targeting the central portions of the mass. Treatment of the tumor boundary is necessary in order to achieve destruction of likely sites of origin of the pain, including nerve endings and involved periosteum ( Fig. 11.1 ). Monopolar multitined electrodes or cool-tip electrodes, or bipolar electrode systems can be used, depending on physician preference and experience and device availability. A single ablation is typically performed for lesions < 3 cm in diameter, and the time of ablation is typically 5 to 10 minutes at the target temperature of 100°C, or until tissue impedance limits energy delivery to the target tissue. For larger lesions, overlapping ablations are performed with the goal of treating the entire bone–tumor interface, with the time of ablation again typically 5 to 10 minutes at the target temperature of 100°C or until tissue impedance limits energy delivery to the target tissue.
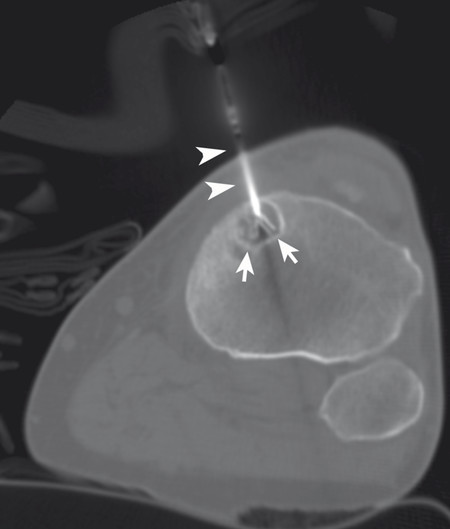
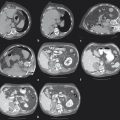
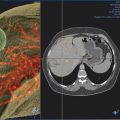
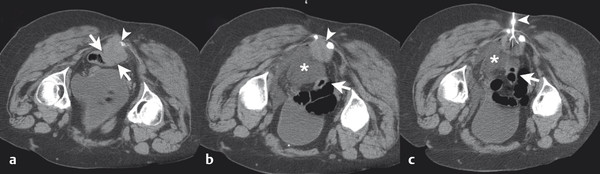
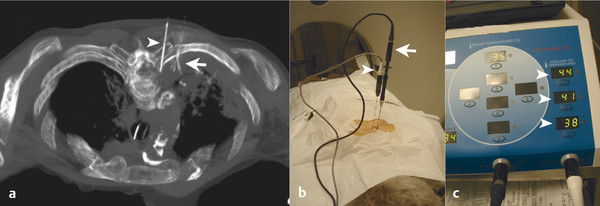
The target tumor should be isolated or adjacent critical structures displaced from the anticipated ablation zone to avoid injury. Multiple methods can be used to reduce the risk of collateral thermal damage of adjacent normal structures. This can typically be accomplished through careful patient positioning or displacement of tissues using fluid (hydrodisplacement), balloons, or gas (carbon dioxide). For example, sterile water (usually 5% dextrose in water [D5W] with the use of RFA to prevent the risk of conduction of energy with buffered fluids) can be injected through a needle to displace loops of bowel away from a target lesion ( Fig. 11.2 ). The fluid can be used with or without the addition of dilute iodinated contrast material (1:50). Thermal monitoring provides feedback during an RFA procedure with placement of a temperature-sensing probe adjacent to a critical structure ( Fig. 11.3 ). Because the margin of the RF ablation cannot be accurately visualized with CT or US imaging (MRI may allow visualization), this thermal monitoring avoids unexpected elevations of temperature and potential injury to adjacent normal structures.
11.5.2 Cryoablation
Cryoablation has emerged as an exceptional treatment method for palliation of painful metastatic disease involving bone and soft tissue outside of the liver and lung. 17 , 18 , 19 , 20 , 21 , 22 Although cryoablation has a long history of successful treatment of neoplasms in various locations in the body, including prostate, kidney, liver, and lung, early systems were not insulated and could only be used in an intraoperative setting. With the advent of well-insulated cryoprobes and the use of Joule–Thomson ports using room-temperature, high-pressure argon gas as a cooling source, percutaneous systems became possible. As the argon gas passes from approximately 3,000 psi in a thin tube within sealed cryoprobes to the relatively larger sealed chamber at the tip of the probe at atmospheric pressure, the gas undergoes rapid cooling to achieve temperatures < − 100°C. At the cellular level, intracellular and extracellular fluid freezes, and tissue destruction results from cell membrane disruption by ice crystals, cellular dehydration, and vascular thrombosis. Temperatures below − 20 to − 40°C are necessary to achieve complete cell death, usually present within 3 to 5 mm of the edge of the ice ball. 23 , 24 Current generation systems can generate an ice ball, using a single probe, of ~ 3.5 cm diameter. Infusion of helium gas into the cryoprobes, which has a Joule–Thomson coefficient opposite that of argon gas, results in tissue heating. Multiple cryoprobes are used simultaneously to generate confluent ice, with the size dependent on the number of cryoprobes, with > 8 cm diameter ice balls readily achieved. The shape of the ablation zone can be controlled through varied geometry of probe placement. Although a freeze–thaw–freeze cycle is necessary to ensure complete cell death, the overall procedure times are not prohibitively long because large or complex tumors can be treated without the time-consuming overlapping ablations needed with other ablation techniques. In addition, in consideration of achieving local tumor control, synchronous ablation with several cryoprobes eliminates residual disease at the ablation interfaces that can result from performing overlapping sequential ablations. 25
Fortunately, the ablation zone that is generated with cryoablation is visualized as a discrete low-attenuation area with noncontrast CT imaging or as a low-signal area with MRI. The edge of the ice ball corresponds to 0°C, and tissue outside this boundary is not at risk for injury. 26 The CT environment is readily available for intervention in most practices, and wide-bore systems are also becoming more common, allowing placement of ablation devices while retaining the ability to image patients without great difficulty. It is possible to image thermal changes with MRI, and some practices are overcoming challenges of performing ablation procedures in this environment while benefiting from improved tumor conspicuity for guidance and ablation monitoring.
Two cryoablation manufacturers produce clinical systems, the Endocare Cryocare System (HealthTronics, Inc.) and the Visual-ICE or SeedNet Cryoablation System (Galil Medical, Inc.). The Endocare System uses two different sizes of insulated cryoprobes measuring 2.4 mm (13-gauge) and 1.7 mm (16-gauge) in diameter. The Galil system employs 1.5 mm (17-gauge; insulated IceRod+ and uninsulated IceSphere and IceSeed) cryoprobes (similar MRI-compatible cryoprobes are available) as well as 2.4 mm IceEdge cryoprobes. The Endocare system has 8 separately controlled channels (8 total), whereas the Galil system has 10 separately controlled channels with two ports on each channel (20 total). These systems generate ice balls of various geometries; for example, the Endocare Perc-24 produces an ice ball up to 3.7 cm in diameter and 5.7 cm in length along the probe shaft.
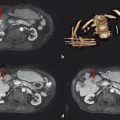
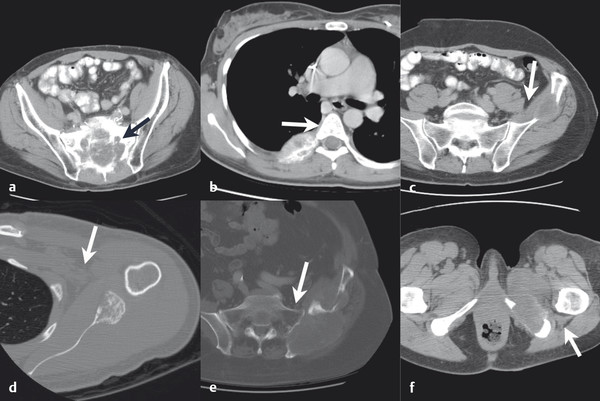
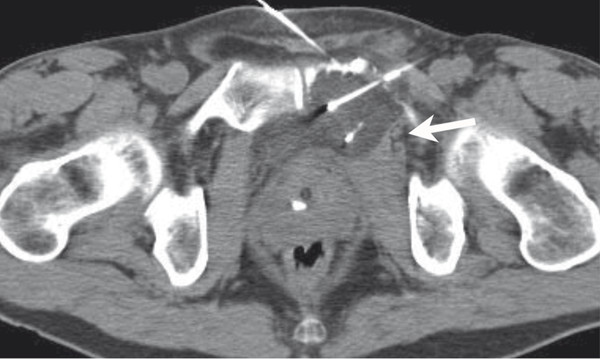
Using typical sterile preparation, cryoprobes are introduced through a skin nick under CT, US, or MRI guidance. Operator experience is necessary to prescribe the placement of cryoprobes, although a general guideline is to place probes within 1 cm of the tumor boundary and at an interprobe spacing of 2 cm within the target tumor. A single freeze–thaw–freeze cycle is performed for each lesion, with typical times for these cycles of 10 minutes–8 minutes–10 minutes, respectively. Shorter or longer times are often used for the freezing portions of the cycle, depending on the adequacy of coverage of the lesion and the proximity of adjacent critical structures. The rate of ice-ball growth can also be controlled by reducing the percentage of time that gas flows through the probes from 100% to as low as 20%. Most commonly, noncontrast CT imaging is performed approximately every 2 to 4 minutes throughout the freezing portions of the cycle, with body window and level settings (W400, L40) to monitor the growth of the ice ball. Typically, the cryoprobes are placed along the long axis of the tumor and at an angle to allow slow growth of the ice in the direction of adjacent critical structures. Identification of adjacent critical structures at the time of the procedure and employment of protective maneuvers is commonly performed. Understanding the path of major motor nerves and the artery of Adamkiewicz is helpful for the performance of safe ablation procedures. 16 As illustrated in Fig. 11.4 , these structures can be clearly identified and avoided at the time of the ablation procedure. An example of the use of cryoablation in the proximity of the obturator nerve is shown in Fig. 11.5 . A soft tissue and bony metastasis involving the pubic bone was treated with four cryoprobes placed through the tumor to generate ice that matched the shape of the complex tumor. The evolution of the ice ball showed complete coverage of the tumor while avoiding the adjacent obturator nerve.
Following completion of the second freeze cycle, cryoprobes are warmed by switching to helium gas until temperatures within the treated tissue are > 20°C. The cryoprobes can be withdrawn at this point, although continued warming of the probes over a period of approximately 10 minutes may result in a reduced risk of hematoma formation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree