Fig. 9.1
Bi-valved block made of tissue-equivalent material with a central chamber to encase the penis during external beam radiotherapy
The clinical target volume (CTV) is the visible/palpable disease with a 1 cm margin. The planning target volume should add another cm to be 2 cm beyond visible/palpable disease to allow for minor set-up variation (larger margin advisable if an opaque block is used) and beam penumbra. Depth of invasion is not a concern as the treated volume includes the full thickness of the penis.
Dose and fractionation have varied over the decades but the currently accepted prescription would be 66–70 Gy over 6.5–7 weeks. Fraction sizes <2 Gy, treatment courses longer than 45 days, and total dose <60 Gy are associated with an increase in local failure [4, 20]. Five-year local control ranges from 41 to 70 % [4, 17, 21–24] with a weighted average of about 61 %.
Penile preservation is approximately the same since most local failures are salvaged surgically with either partial or total penectomy. Results from selected series are presented in Table 9.1.
Table 9.1
Published results for external beam radiotherapy
Author | n | Fup(m) | Dose Gray | CSS | DFS | LC | compln | Penile preservn | |
|---|---|---|---|---|---|---|---|---|---|
Neave et al. [23] | 1993 | 20 | 36+ | 50–55/20–22 | 58 % | – | 70 % | 10 % sten | – |
McLean et al. [17] | 1993 | 26 | 116 | 35/10–60/25 | 69 % | 15/26 | 62 % | 27 % unspec | 100 % |
Ravi et al. [64] | 1994 | 128 | 83 | 50–60 | – | 84 % | 65 % | 6 % nec | – |
24 % sten | |||||||||
Sarin et al. [4] | 1997 | 59 | 62 | 50–60 | 66 % | – | 35/59 | 3 % nec | 55 % |
14 % sten | |||||||||
Gotsadze et al. [24] | 2000 | 155 | 40 | 40–60 | 88 % | 65 % | 1 % nec | 65 % | |
7 % sten | |||||||||
Zouhair et al. [20] | 2001 | 23 | 70 | 45–074 @ 1.8–2 Gy | – | 57 % | 10 % sten | 36 % | |
Ozsahin et al. [65] | 2006 | 33 | 62 | 52 | 53 % 10y | – | 44 % | 10 % sten | 52 % |
Azrif et al. [21] | 2006 | 41 | 54 | 50–52/16 | 96 % | 51 % | 62 % | 8 % nec | 62 % |
29 % sten | |||||||||
Mistry et al. [22] | 2007 | 18 | 62 | 50/20–55/16 | 85 % | 63 % | 63 % | 2 nec | 66 % |
1 sten |
External beam radiotherapy is most frequently considered in very elderly or debilitated patients or those presenting with loco-regionally advanced disease where the primary would be treated in contiguity with the nodal regions, including both groins and the pelvis. This will be addressed further under Regional Radiotherapy.
Interstitial Low Dose Rate (LDR) Brachytherapy
The penis lends itself well to the application of interstitial brachytherapy and has been successfully treated with LDR brachytherapy for decades with reports from Europe, India, and Canada. Interstitial brachytherapy can deliver the required dose precisely and accurately to the target without excessive treatment of the penile shaft or concerns about daily set-up. Furthermore, for well lateralized lesions it is not necessary to treat the full thickness of the penis and some sparing of the contra-lateral glans is possible [25]. Technique and dose prescription have been more consistent over time with LDR brachytherapy than in the external beam literature.
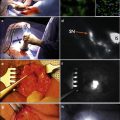
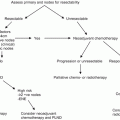
LDR interstitial brachytherapy is performed with sterile technique in a dedicated brachytherapy procedure room or operating room under general anesthesia or penile block. The procedure generally takes 45 min to 1 h with the patient admitted afterward for the duration of the brachytherapy (4–6 days). After skin cleansing and creation of a sterile field, the penis is examined carefully and the visible/palpable lesion is delineated with sterile pen and the appropriate margins chosen. The patient is catheterized and an in-dwelling Foley catheter is left in situ until completion of treatment and removal of the brachytherapy needles. The position and spacing of the interstitial needles is chosen so as to avoid the urethra, and to have the superficial needles within 3 mm of the treated surface. If they are too deep, the surface will be underdosed, while if they are too shallow, skin ulceration and scarring will result. A minimum of a two-plane implant is required (Fig. 9.2). The needle direction can be either antero-posterior for a lateral lesion, or right–left for a thicker infiltrating lesion. If the anatomy does not allow both avoidance of the urethra and optimal depth under the surface, then a plesiotherapy plane can be added externally on the side of the cancer with the air gap filled with appropriate bolus material such as “superflab”, available from several radiotherapy supply companies (Fig. 9.3).
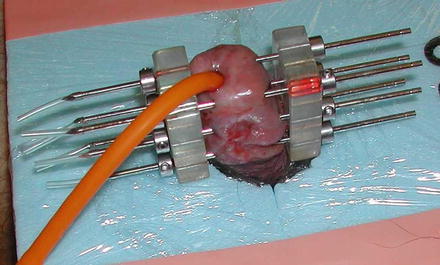
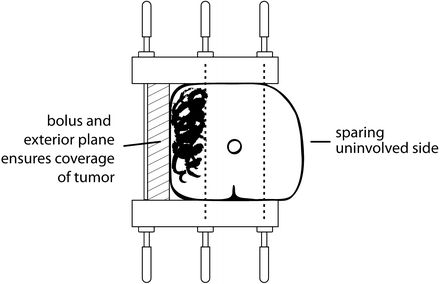

Fig. 9.2
Two-plane implant showing Foley catheter and styrofoam plaque to support penis and distance the treated area from the normal tissues

Fig. 9.3
Schematic of a three-plane implant showing a lateral plane of needles exterior to the penis to supply plesiotherapy, with a layer of tissue-equivalent bolus filling the gap. The depth of needles on the uninvolved side allows some sparing of the skin surface on that side. (Reproduced with permission from [25])
Guide templates are required to ensure parallelism and equal spacing. Both the needles and planes should be equidistant in an LDR implant. Ideal spacing is 12–18 mm with 15 mm most commonly used. Pairs of templates should be available, predrilled with an appropriate range of needle spacing but a “universal template” with holes drilled every 3 mm allows more flexibility once the first couple of needles have been placed (Fig. 9.4). Once positioned, the needles are fixed in place with washers against the template with a single set-screw in each to tighten against the shaft of the needle. Since the implant geometry is totally stable throughout the duration of the treatment, dosimetry can be calculated based on measurements of spacing and treated lengths but more commonly a CT scan is performed and the needles reconstructed from the scan.
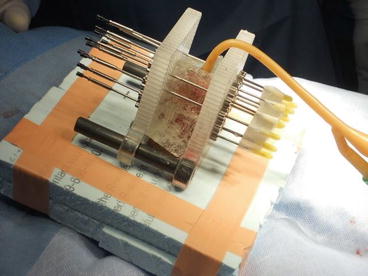

Fig. 9.4
“Universal” template with holes drilled every 3 mm, so spacing can be selected as suitable at 9, 12, 15 mm, etc. Nine to twelve millimeter would be suitable for an HDR implant while 15 mm is the preferred spacing for LDR brachytherapy. A layer of superflab bolus can be seen
The basic rules of geometry of the Paris System of Dosimetry should be appreciated in order to place the needles optimally [26]. When using classic LDR treatment with Iridium wire, it is essential to be aware that the length of your treated volume along the axis of the needles is 0.75 of the active length of the wire sources and to allow for the consequent in-drawing of the isodoses between the ends of the wires. Similarly, the spacing between the needles determines the lateral margin treated beyond the sources (0.27 × spacing; i.e.: 4 mm for 15 mm spacing; Fig. 9.5). If a stepping source is used from an automated afterloader, such as in Pulse Dose Rate Brachytherapy, then dose optimization is possible. Nonetheless, the prescribing rules of the Paris System give guidance as to desirable homogeneity, such that dose rate minima between the sources are 115 % of the prescription isodose, and the sum of the high dose sleeve around each source (V200) is <10 % of the treated volume.
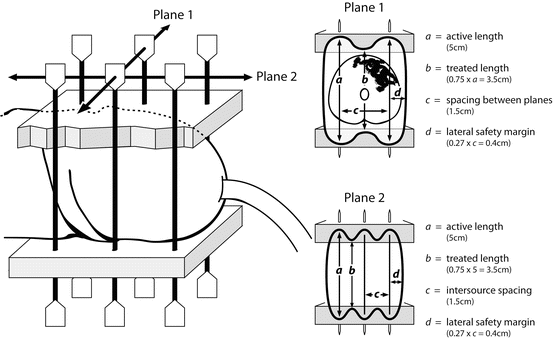

Fig. 9.5
Schematic of a two-plane interstitial implant typical of LDR brachytherapy and adhering to the Paris Rules of Dosimetry for geometry and needle spacing. In-drawing of the prescription isodose between the ends of the sources is represented by “b” and the lateral margin around the prescription isodose is shown as “d”. (Reproduced with permission from reference [25])
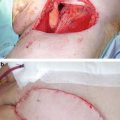
Classic continuous LDR brachytherapy aims for a dose rate of 50–60 cGy per hour. Pulse dose rate brachytherapy (Fig. 9.6) is radiobiologically equivalent if hourly fractions of 0.5–0.6 Gy are delivered, 24 h per day [27–29]. Most PDR results are mixed with classic LDR in single institution reports but Kamsu-Kom et al. from Institut Gustav Roussy recently reported on 27 patients treated from 2008 to 2013 exclusively with PDR brachytherapy to a dose of 60–70 Gy with 0.4–0.5 Gy per hour, with results indistinguishable from those reported with classic LDR [30]. The total dose recommended is generally 60–65 Gy over 5 days. Minimal analgesia is required during the duration of the implant and the patient can even mobilize within the hospital room, disconnected from the afterloader between fractions for PDR cases. If the patient is less mobile, then anti-embolic stockings and low molecular weight heparin are advised as anti-thrombotic measures. Needle removal can occur at the bedside after premedication with a narcotic analgesic such as Demerol or morphine. Bleeding is usually minimal and the patient can be discharged the same day.
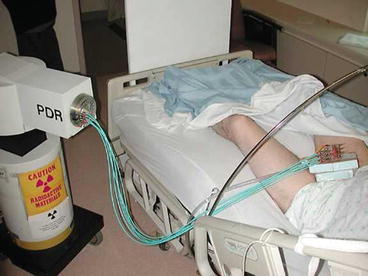

Fig. 9.6
Patient connected to Pulse Dose Rate afterloader with a transfer tube attached to each interstitial needle
Selected results from the literature for interstitial brachytherapy are shown in Table 9.2. Five-year local control ranges from 70 to 96 % and 10-year from 70 to 80 %, with penile preservation at 10 years being 70 %. Local failures can be salvaged surgically and, as they can occur late, up to 8–10 years after treatment, continued surveillance and patient-awareness are essential. Crook et al. found that although five of eight local recurrences occurred in the first 2 years, the remaining three occurred at 4.5, 7, and 8 years after brachytherapy [18]. Similarly, Mazeron et al. [31] reported that 18 % of local recurrences occurred between 5 and 8 years and DeCrevoisier et al. found that with longer follow-up, 20 % were diagnosed beyond 8 years [19].
Table 9.2
Published results for brachytherapy
Author | Year | HDR LDR | n | Fup(m) (range) | Dose Gray | CSS | DFS | LC | compln | Penile preservn |
|---|---|---|---|---|---|---|---|---|---|---|
Mazeron et al. [31] | 1984 | LDR | 50 | 36–96 | 60–70 | 79 % | 63 % | 78 % | 3 % nec | 74 % |
16 % sten | ||||||||||
Delannes et al. [46] | 1992 | LDR | 51 | 65 (12–144) | 50–65 | 85 % | – | 86 % | 23 % nec | 75 % |
45 % sten | ||||||||||
Rozan et al. [40] | 1995 | LDR | 184 | 139 | 59 | 88 % 5y | 78 % 5y | 85 % | 21 % nec | 76 % |
88 %10y | 67 %10y | |||||||||
45 % sten | ||||||||||
Soria et al. [5] | 1997 | LDR | 102 | 111 | 61–70 | 72 % 5y | 56 % 5y | 89 % | 1 nec | 68 % |
1 sten | ||||||||||
66 %10y | 42 %10y | |||||||||
Chaudhary et al. [47] | 1999 | LDR | 23 | 24 (4–117) | 50 (40–60) | – | – | 70 % | 0 nec | 70 % |
2/23 sten | ||||||||||
Kiltie et al. [39] | 2000 | LDR | 31 | 61.5 | 63.5 | 85 % | 85 % | 81 % | 8 % nec | 75 % |
44 % sten | ||||||||||
de Crevoisier et al. [19] | 2009 | LDR | 144 | 68 (6–348) | 65 | 92 %10y | 78 %10y | 80 %10y | 26 % nec | 72 %10y |
29 % sten | ||||||||||
Crook et al. [18] | 2009 | LDR | 67 | 48 (44–194) | 60 | 84 % | 71 %5y | 87 %5y | 12 % nec | 88 %5y |
72 %10y | ||||||||||
9 % sten | 67 %10y | |||||||||
Pimenta et al. [66] | 2015 | LDR | 25 | 110 (0–228) | 60–65Gy | 91 % | 92 %5y crude | 1 LF @ 4 months | 8 % nec | 86 %(5y) |
43 %sten | ||||||||||
Petera et al. [33] | 2011 | HDR | 10 | 20 | 3 Gy bid = 42–45 | 100 % | NS | 100 % | 0 nec | 100 % |
0 sten | ||||||||||
Sharma et al. [32] | 2014 | HDR | 14 | 22 (6–40) | 3 Gy bid = 51 Gy | 83 % 3y | NS | 12/14 | 0 nec | 93 % |
0 sten | ||||||||||
Rouscoff et al. [36] | 2014 | HDR | 12 | 27 (5–83) | 36/9–39/9 bid | 100 % | 83 % | 11/12 | 1/12 nc | 11/12 |
1/12 sten | ||||||||||
Kellas-Sleczka et al. [34] | 2015 | HDR | 55 | 55 (8–154) | 3–3.5 bid = 30–54 | NS | NS | 73 % | 0 nec | 80 % |
0 sten |
High-Dose Rate Interstitial Brachytherapy
The use of manually afterloaded sources such as Iridium 192 wires is no longer available in most departments due to reasons of staff exposure, logistics of source disposal after use, and risks of contamination from having to cut brittle wire to the correct length. On the contrary, HDR afterloaders are available in most radiotherapy centers for use in more common malignancies such as breast and prostate, and as an essential component of curative treatment of cervical cancer. Recently, there has been renewed interest in brachytherapy for penile cancer using HDR. The technique is very similar to that described above for LDR but with a few notable exceptions.
- 1.
Needle spacing should be closer than for LDR since HDR is less forgiving of “high dose sleeves” around sources. Ideal spacing is 9–12 mm.
- 2.
Needle spacing does not have to be equidistant as variations in spacing are easily compensated for with the stepping source.
- 3.
A “universal template” with holes every 3 mm is ideal since the spacing around the urethra can be 12 mm and elsewhere reduced to 9 mm.
- 4.
Attention to homogeneity is very important. The desired parameters are still being established.
Recent publications indicate that 3 Gy bid, 6 h apart is safe and effective for a total dose of 45–51 Gy delivered over 7.5–8.5 days [32, 33]. Penile preservation rates are reported between 80 and 100 %. Kellas-Slezka et al. report the largest series of 55 patients with median follow-up of 4.5 years [34]. A freehand flexible catheter technique was used to place 2–7 catheters to create either a single plane implant (n = 31) or two-plane (n = 24). Fraction size was 3.0–3.5 Gy with a median total dose of 49 Gy after biopsy (range 30–54 Gy) or 36 Gy (range 30–45.5) after previous total gross excision. Median duration of implant was 11 days and median follow-up was 59 months (8–152 months). Persistent tumors were seen in four patients and seven had a subsequent local recurrence. Regional failures were seen in 22 % and distant metastases in 5 %. Penile preservation was achieved in 80 % [34]. Although this is a large series with mature follow-up, at the time of writing, HDR brachytherapy for penile cancer must still be considered to be in evolution, as optimal fractionation and homogeneity parameters are yet to be established.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree