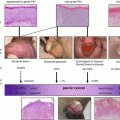
Fig. 8.1
Overall survival stratified by receipt of adjuvant chemotherapy in chemotherapy-naïve men with pelvic node-positive penile cancer. (Reproduced, with permission from Sharma et al. [21])
The summary of evidence on adjuvant chemotherapy in node-positive penile cancer is shown in Table 8.1. Taken together, there does appear to be a role for adjuvant platinum-based therapy for chemotherapy-naïve patients with pN3 penile cancer. There are no randomized prospective data, however, and reported follow-up is short, raising questions on whether a durable disease-free survival can be achieved. Additionally, the optimum regimen (platinum-based triplet or doublet) has not been defined, and all of the tested regimens appear to carry at least moderate toxicity. The lack of unequivocal evidence is reflected in the discordance between the current European Association of Urology (EAU) and National Comprehensive Cancer Network [NCCN] guidelines [22, 23], with the former advocating upfront surgery and the use of adjuvant chemotherapy in pN2-3 disease, and the latter recommending neoadjuvant chemotherapy based on clinical staging.
Table 8.1
Summary of studies on adjuvant chemotherapy in node-positive penile cancer
Citation | Patient cohort | N | Regimen(s) | Median (mean) follow-up, months | Survival outcomes | Toxicity |
|---|---|---|---|---|---|---|
Pizzocaro and Piva [18] | Involved inguinal and/or pelvic nodes | 12 | VBM | 42 | 11 alive and disease-free | Bleomycin-induced lung damage (n = 2) |
1 died of disease | ||||||
Hakenberg et al. [8] | pTx pN1-3 M0 | 8 | BMP | (54) | 3 alive and disease-free | Treatment-related death (n = 1) |
4 died of disease | Any grade 3 or 4 (n = 24/45) | |||||
Noronha et al. [20] | High-risk nodal disease | 19 | TP | 15 | 6 loco-regional relapses | Treatment-related death (n = 1) |
2 died of disease | Any grade 3 or 4 (n = 6) | |||||
Nicolai et al. [19] | ≥pN2 M0 | 19 | TPF (n = 16) | n/a | 10 alive and disease-free | Any grade 3 or 4 (n = 10) |
8 died of disease | ||||||
1 died of other cause | ||||||
2-year DFS of 37 % | ||||||
Paclitaxel-PF (n = 3) | ||||||
Sharma et al. [21] | pN3 M0 | 36 | TPF (n = 18) | 12 | mOS = 21.7 months (versus 10.1 months in 48 men who did not receive adjuvant chemotherapy, p = 0.048) | n/a |
PF (n = 8) | ||||||
VBM (n = 8) | ||||||
TIP (n = 1) | ||||||
BMP (n = 1) |
Role of Postoperative Radiotherapy in Node-Positive Disease
There is a paucity of data on the role of a djuvant radiotherapy for men with resectable nodal disease who undergo inguinal lymphadenectomy. Yet, despite this, adjuvant radiation is widely used in several European countries to manage regionally metastatic disease, whereas population-based data have shown that it is much less commonly utilized in the US, with less than one in six men who undergo a lymph node dissection receiving adjuvant radiation [24].
Chen and colleagues from Taiwan reported an 11 % regional recurrence rate with adjuvant radiotherapy for men with pathologic inguinal lymph node metastasis, compared to 60 % for men who did not receive any radiotherapy; however, the overall number of patients was very small (n = 14) and no details were reported on the pathologic node characteristics (e.g., extranodal extension) [25]. A similar experience from Leeds, United Kingdom, showed a rate of regional relapse in 6 of 14 men (43 %) treated with adjuvant radiation, including 3 of 4 patients with extranodal extension (pN3) [26]. The authors commented that the 3-year overall survival of 32 % observed in their cohort with pN3 disease was favorable in comparison to a corresponding figure of 18 % that was reported in a surgical series from India [27]. The evidence from these very small series is inconclusive.
Adding to this uncertainty are data from an analysis utilizing the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) Medicare-linked database, which abstracts data from approximately 28 % of the US population. In this study, Burt and colleagues analyzed stage at presentation and treatment outcomes in 2458 patients with squamous cell cancer of the penis, and noted that adjuvant radiation was not associated with cause-specific survival on a multivariate analysis adjusting for tumor stage, grade, and receipt of lymphadenectomy (HR = 1.09 [0.74–1.61], p = 0.65) [24].
In summary, there is limited evidence in favor of postoperative radiotherapy for prophylaxis in node-positive penile cancer, and its use is controversial. While a few case series have postulated a survival benefit, particularly in pN3 disease, the data are from extremely small numbers of patients. Larger series or prospective studies are needed before its use can receive an evidence-based recommendation.
Multimodal Approach to Bulky, Fixed, or Unresectable Nodal Disease
There is good-quality evidence suggesting that surgery alone is not a curative option in men with advanced inguinal or pelvic nodal disease; bilateral, numerous and bulky inguinal involvement, extranodal extension, and the presence of pelvic nodal metastases are well-known prognostic factors in penile cancer, and the use of a multimodal approach in these patients is desirable [3, 27, 28].
The earliest significant study attempting to evaluate initial chemotherapy for metastatic disease was the Southwest Oncology Group (SWOG) phase II evaluation of bleomycin, methotrexate, and cisplatin (BMP) in 45 men with locally advanced or metastatic penile cancer. Although a response was seen in almost one in three evaluable patients, this did not translate into a durable survival benefit, with a median overall survival of only 28 weeks. More importantly, there was a significant toxicity burden, with five treatment-related deaths (due to infection or pulmonary complications) and nearly one in three men suffering grade 4 toxicity of any kind [29].
Although these early results were disappointing, they have instigated further study on optimizing a multimodal approach to treating locally advanced disease. Similarities between squamous cell carcinomas of the penis and head and neck have led investigators to study other neoadjuvant chemotherapy regimens. Leijte and colleagues from the Netherlands reported the outcomes for 20 patients who received neoadjuvant chemotherapy for M0 penile cancer between 1972 and 2005 [7]. The regimens were heterogeneous, including VBM and BMP, in addition to single-agent bleomycin or irinotecan, and an objective response (either complete or partial response) was seen in 12 of 19 evaluable patients (63 %). Notably, nine responders went on to undergo lymphadenectomy (with two found to be in pathologic complete response (pCR) on postoperative histology), and eight of these men had durable long-term survival with no evidence of disease at a median follow-up of 20 months. Response to neoadjuvant therapy was also prognostic, with a 5-year overall survival of 56 % in those who responded, while all non-responders had died within 9 months of treatment. As seen with the SWOG and adjuvant chemotherapy studies, there was notable toxicity, with three treatment-related deaths (two men who received BMP and one who received VBM).
Italian investigators from Milan have also reported retrospective data on their experience, the overall results of which appear to be slightly poorer compared to the Dutch data. A median of four cycles of a taxane (paclitaxel or docetaxel) in combination with cisplatin and 5-fluorouracil was given in the neoadjuvant setting to 28 men with clinical N3 disease, producing an overall response rate of 43 % [19]. Seven of the 22 men (32 %) who went on to undergo surgery were alive and disease-free at a median follow-up of more than 12 months, including two of the four who achieved a pCR. Overall, 12 of the 28 men relapsed, with nine dying of disease, and another dying of treatment-related cardiac toxicity. Although response to treatment was not associated with survival in this dataset, the study was underpowered for this, and overall, these retrospective European data serve to confirm that a multimodal approach involving neoadjuvant platinum-based chemotherapy is feasible and has the potential to achieve long-term remissions.
Four prospective studies have bolstered the evidence base on neoadjuvant chemotherapy for locally advanced disease, although the small numbers of patients involved make it difficult to generate robust conclusions. A phase II study of irinotecan in combination with cisplatin was performed by the European Organization for Research and Treatment of Cancer (EORTC) and reported in 2008 [11]. Seven men in that study had T3 and/or N1/N2 disease and were treated in the neoadjuvant setting, with two (29 %) achieving a partial or complete response, and three (43 %) achieving a pCR at lymphadenectomy. This report did not provide any survival data, but again adds to the body of evidence on the feasibility of a multimodal approach to locally advanced penile cancer.
The largest prospective study on neoadjuvant chemotherapy for locally advanced disease was a phase II trial conducted at the University of Texas MD Anderson Cancer Center [10]. Thirty men with clinical stage Tx N2-3 M0 disease were given four cycles of paclitaxel, ifosfamide, and cisplatin (TIP) prior to undergoing planned bilateral inguinal and uni- or bilateral pelvic lymph node dissection. Most of the patients (70 %) had clinical N3 disease at baseline, and the majority completed all four cycles with minimal toxicity (grade 3 infection was the most commonly observed adverse event, occurring on five occasions), with 15 of 30 men (50 %) achieving an objective response. All but four individuals went on to undergo lymphadenectomy, including 22 of the 23 men who completed all four cycles of chemotherapy, with 3 of these 22 men (13.6 %) being found to have a pCR. Survival data were comparable to that observed in previous retrospective studies [7, 19], with nine men (30 %) remaining alive and without evidence of disease at a median follow-up of 34 months; median overall survival in the entire cohort was 17 months. Importantly, response to neoadjuvant TIP was significantly associated with a longer overall survival and time to disease progression (Fig. 8.2), while postoperative complications were comparable to those seen in contemporary lymphadenectomy series [30].
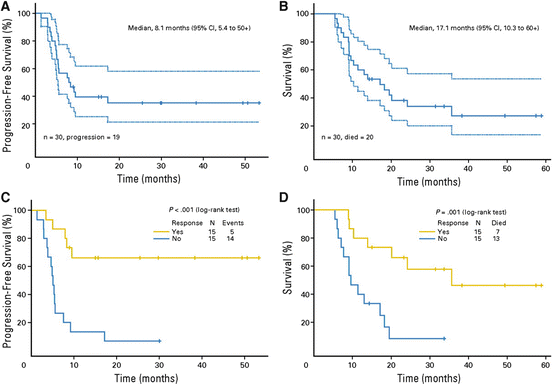

Fig. 8.2
Kaplan–Meier curves showing (a) time to disease progression and (b) overall survival in men receiving neoadjuvant TIP, and (c, d) time to progression and overall survival stratified by response to neoadjuvant chemotherapy respectively. The 95 % CI are shown (a, b) with dashed lines above and below. (Reproduced, with permission from Pagliaro et al. [10])
A follow-up report from the same group expanded on this phase II trial by retrospectively reviewing results from an additional 31 men with Tx N1-3 M0 disease who underwent neoadjuvant chemotherapy, to produce an overall cohort of 61 patients, 21 of whom had undergone a prior inguinal procedure and were being treated for disease recurrence or persistence [31]. The majority of this 61-man cohort received TIP and an impressive overall response rate of 65 % (39 of 61 patients) was observed. Fifty-two patients (85 %) went on to undergo surgery, with ten (19 % of those operated) achieving a pCR. Twenty men (33 %) were alive and disease-free at a median follow-up of more than 5 years, including seven of the ten who achieved a pCR, and overall, 50 % of men who responded to neoadjuvant therapy were alive at 5 years.
Prospective studies of other platinum-based regimens in the treatment of metastatic penile cancer were not as successful as the MD Anderson experience with TIP [10]. Nicholson et al. administered a median of three cycles of TPF to 21 men with Tx N1-3 M0 disease, including 15 (71 %) with N3 disease, as part of a phase II clinical trial in the UK [32]. They aimed to assess response clinically and via RECIST after the planned three cycles of chemotherapy, and determine how many patients were subsequently operable. An overall response was seen in 7 of 19 evaluable patients (37 %) in this neoadjuvant group, and of the 20 patients deemed inoperable at trial entry, 5 were sufficiently downstaged to be operable, although one was considered too frail to undergo surgery. Notably, more than two in three patients suffered any grade 3 or 4 toxicity, which was substantially greater than that observed with neoadjuvant TIP [10].
The latest prospective data to examine the role of neoadjuvant chemotherapy come from a recent report from The Netherlands Cancer Institute [12]. This group reported the outcomes of 26 men with T4 and/or N3 disease who were treated with neoadjuvant TPF as part of a nonrandomized institutional study, with the dose of cisplatin used being higher than that in the aforementioned UK TPF trial [32]. As with the previous study, they aimed to achieve sufficient downstaging to permit surgical resection with curative intent. Almost half of the cohort completed all of the planned four cycles of therapy, with 11 of the 25 evaluable patients (44 %) achieving at least a partial radiographic response. Fourteen men underwent surgery, and a pCR was seen in one (7 %), similar to the pCR rate in the MD Anderson study of neoadjuvant TIP [10]; however, only four men (15 % of the entire cohort) were alive and disease-free at a median follow-up of 30 months. The median overall survival was 10 months, which was disappointingly lower than the 17 months seen with neoadjuvant TIP, although it must be noted that the Dutch cohort was heterogeneous, with almost half being treated for recurrent disease, which may have selected for more aggressive disease biology. Additionally, six men discontinued therapy owing to toxicity, while all enrolled men experienced some grade of chemotherapy-related toxicity.
A synthesis of published evidence of the use of neoadjuvant chemotherapy for locally advanced penile cancer is shown in Table 8.2. It is apparent that adopting a multimodal approach to treat locally advanced disease is feasible, and response rates to neoadjuvant chemotherapy of between 29 and 65 % have been seen with retrospective and prospective data. Platinum-based therapy has emerged as the standard of care, with TIP offering the highest response rates. While all studies generally demonstrate a reasonable response rate, the difference in durable survival benefit is less clear. Current EAU [22] and NCCN [23] guidelines do recommend neoadjuvant chemotherapy followed by consolidation surgery in responders for nodal disease that is initially bulky or unresectable, with a triplet regimen, containing cisplatin and a taxane, preferred wherever feasible.
Table 8.2




Summary of studies on neoadjuvant chemotherapy for unresectable nodal disease
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree