Sun-deprivation
The vitamin D specifications apply to individuals with minimal or no sunlight exposure. This encompasses housebound individuals especially the frail elderly, those who practice concealment for cultural or religious reasons, those with darker skin, those that apply high factor sunscreen, and those residing in high-latitude countries during the months when there is absent skin generation of vitamin D. These otherwise healthy individuals are at risk of reduced vitamin D synthesis.
Dietary reference intakes (DRIs)
Estimated average requirement (EAR): meets the needs of 50 % of the population. The EAR is an appropriate estimate when considering intake for groups or persons.
The recommended daily allowance (RDA) meets the needs of over 97.5 %. The RDA is likely an overestimate of need for any particular individual; but since the true requirement of an individual may not be known, the clinician may aim for this higher intake level.
Vitamin D status as judged by serum or plasma 25OHD level
25OHD is considered a “biomarker of exposure” (namely, the best measure of vitamin D supply) but it is not a “biomarker of effect” (namely, it is not a clinical outcome).
The plateau of skeletal benefit is reached at 30–40 nmol/L (12–16 ng/ml).
The EAR corresponds to a 25OHD level of 40 nmol/L (16 ng/ml).
The RDA corresponds to a 25OHD level of 50 nmol/L (20 ng/ml).
Current vitamin D status in USA
In the USA, the median oral intake of vitamin D is less than 400 IU/d but the mean 25OHD levels are above 50 nmol/L. The 25OHD level is higher than expected for vitamin D intake; this suggests, not surprisingly, that supply from sun-light exposure either inadvertent or intentional contributes substantially to vitamin D status. This reinforces the point that the EAR and RDA apply to sun-deprived individuals.
Safe vitamin D intake level and safe 25OHD level
The tolerable upper intake level is defined by the IOM report as the upper level of vitamin D intake beyond which harm could be expected to increase for the general population. The IOM specified that this threshold is 4,000 IU daily, and also specified that this not to be considered as a target intake. Furthermore, IOM specified that a 25OHD level of 125 nmol/L (40 ng/ml) corresponds with this upper intake level.
Calcium intake
The clinician must also consider the EARs and RDAs for calcium intake.
Privational vitamin D deficiency is best defined as a clinical, biochemical, radiologic, densitometric or histomorphometric abnormality that is corrected and prevented by low dose vitamin D supplementation [9]. The natural history of vitamin D-related bone disease at the bone level is a phase of secondary hyperparathyroidism (SHPT) with accelerated irreversible bone loss culminating in a mineralization defect (rickets or osteomalacia). Once the entire surface of bone is covered in unmineralized bone matrix (osteoid), irreversible bone loss ceases [10]. On the contrary for hypophosphataemic bone disease, the natural history is one of progressive mineralization defect [11]. This understanding is important in addressing differential diagnosis (Fig. 23.1).
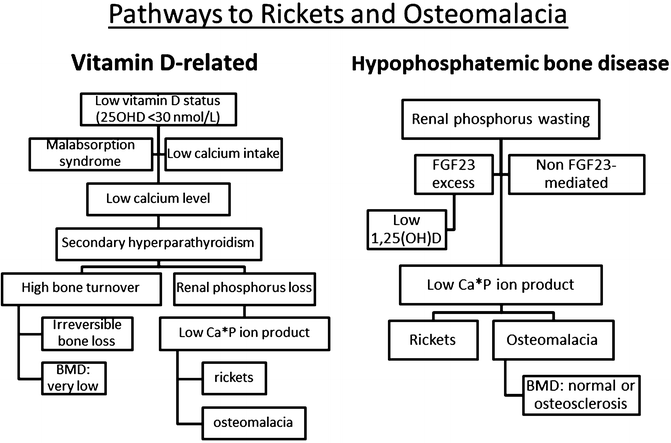

Fig. 23.1
BMD = bone mineral density; FGF23 = fibroblast growth factor 23; Ca = calcium; P = phosphorus; 25OHD = 25-hydroxyvitamin D; 1,25(OH)2D = 1α,25-dihydroxyvitamin D
Measuring 25OHD
Serum or plasma 25OHD is the best measure of vitamin D status because its synthesis is substrate dependent and it has a long half-life of about 2 weeks [12]. There are two types of assay for detecting total 25OHD, 25OHD3 and 25OHD2: (1) immunoassays and automated immunoassays for total 25OHD; and (2) high pressure liquid chromatography (HPLC) liquid chromatography tandem mass spectrometry (LC-MS/MS) and isotope dilution liquid chromatography tandem mass spectrometry (ID-LC-MS/MS) for 25OHD3 and 25OHD2.
One of the major factors contributing to analytical uncertainty in 25OHD testing is the lack of standardization of 25OHD methods [13–16]. Intermethod variability should improve following the introduction in 2009 of Standard Reference Materials (SRM 972) and solvent-based primary calibrators (SRM 2972) by the American National Institute of Standards and Technology (NIST), and also following the acceptance by the Joint Committee for Traceability in Laboratory Medicine (JCTLM) of the NIST and University of Ghent assays (ID-LC-MS/MS and ID/LC/MS) as reference measurement procedures (RMPs) [17]. However, 3 of the 4 SRM 972 reference materials are either spiked with exogenous metabolites (Level 3 with 25-hydroxyvitamin D2, and Level 4 with 3-epi-25-hydroxyvitamin D3) or diluted in horse serum (Level 2) which makes these levels unsuitable for many immunoassays. Only the SRM 972 Level 1 pool should be used for standardization purposes in immunoassays [18]. A new generation of human serum-based SRMs are due to be released that should further improve assay standardization.
Many of the automated immunoassays, which do not have preliminary solvent extraction or protein precipitation to free 25OHD from vitamin-D-binding proteins (DBPs), are subject to DBP matrix interferences [16]. Also for immunoassay techniques, a measure of total metabolite concentration and equivalent detection of both 25OHD2 and 25OHD3 is challenging, because binding proteins show a higher affinity for 25OHD3 than 25OHD2 [19]. All immunoassays have a high cross-reactivity with the metabolite 24,25-dihydroxyvitamin D, which can be present in serum at concentrations of up to 12 nmol/L [20]. LC-MS/MS methods have been shown to suffer from two interferences: the C-3 epimer of 25OH D3, and isobaric substance 7-α-hydroxy-4-cholesten-3-one [21, 22]. The NIST standard containing 3-epi-25OHD3 (SRM 972 Level 4) allows laboratories to check whether or not their method suffers from interference from this metabolite. The isobaric substance has been separated by a novel LC-MS/MS method [21].
It is challenging for clinicians to assess multiple 25OHD results for a given patient if performed at different laboratories using different methods of measurement [23]. It is important for clinicians to be provided by their 25OHD service providers with their assay limitations with regard to traceability, specificity, imprecision and limit of detection. Their participation in a proficiency testing scheme such as the International Vitamin D External Quality Assessment Scheme (DEQAS) is essential [24]. Clinicians should be alerted to any change of methodology as this could have a significant impact on results, patient classification, and treatment recommendations. Finally, clinicians need to ignore reference ranges for 25OHD from commercial laboratories that quote inordinately high levels for vitamin D status [7].
IOM and Defining Vitamin D Status
Vitamin D status should be considered in the light of the recent IOM report, which revised the dietary reference intakes (DRIs) for the USA and Canada (Table 23.1). The 2011 IOM report is now the standard on vitamin D requirement and on vitamin D status because it examined the totality of evidence with respect to harms and benefits for both calcium and vitamin D for the entire population [8]. Using a risk assessment framework they specified the estimated average requirement (EAR) that meets the need of approximately 50 % of the population, and the recommended daily allowance (RDA) that meets the need of 97.5 % of the population (Table 23.2). They specified that a 25OHD level of 40 nmol/L (16 ng/ml) corresponded to the EAR and that a level of 50 nmol/L (20 ng/ml) corresponded to the RDA [3, 7, 25].
Table 23.2
Dietary reference intakes for calcium and vitamin D as specified by 2011 IOM report
Life stage group | Calcium mg/d | Vitamin D IU/d | ||||
|---|---|---|---|---|---|---|
EAR | RDA | UL | EAR | RDA | UL | |
Infants 0–6 months | * | * | 1,000 | ** | ** | 1,000 |
Infants 0–12 months | * | * | 1,500 | ** | ** | 1,500 |
1–3 years old | 500 | 700 | 2,500 | 400 | 600 | 2,500 |
4–8 years old | 800 | 1,000 | 2,500 | 400 | 600 | 3,000 |
9–13 years old | 1,100 | 1,300 | 3,000 | 400 | 600 | 4,000 |
14–18 years old | 1,100 | 1,300 | 3,000 | 400 | 600 | 4,000 |
19–30 years old | 800 | 1,000 | 2,500 | 400 | 600 | 4,000 |
31–50 years old | 800 | 1,000 | 2,500 | 400 | 600 | 4,000 |
51–70 years old | 800 | 1,000 | 2,000 | 400 | 600 | 4,000 |
51–70-year-old females | 1,000 | 1,200 | 2,000 | 400 | 600 | 4,000 |
71+ years old | 1,000 | 1,200 | 2,000 | 400 | 600 | 4,000 |
14–18 years old, pregnant/lactating | 1,100 | 1,300 | 3,000 | 400 | 600 | 4,000 |
19–50 years old, pregnant/lactating | 800 | 1,000 | 2,500 | 400 | 600 | 4,000 |
The implications of the IOM report for clinical practice are summarized in Table 23.1. The IOM report avoided using the terms “vitamin D deficiency” and “vitamin D insufficiency” when defining vitamin D status. Appropriate terms included “hypovitaminosis D” or “low vitamin D status” for a result below 30 nmol/L (12 ng/ml); and “vitamin D adequacy” or “vitamin D sufficiency” for levels 30–50 nmol/L (12–20 ng/ml).Just as there is a range of requirement for vitamin D intake, so is there a corresponding range of adequacy or sufficiency for 25OHD [7]. The RDA and the corresponding 25OHD of 50 nmol/L (20 ng/ml) is likely an overestimate of the need for any particular individual; but since the true requirement of an individual may not be known, the clinician may aim for this higher 25OHD level in defining adequacy or sufficiency [7, 8]. The IOM report expressed concern about levels above 125 nmol/L (50 ng/ml) based on emerging evidence about risks that could not be defined in the usual terms of vitamin D toxicity.
Secondary Indices of Vitamin D Deficiency (Fig. 23.2)
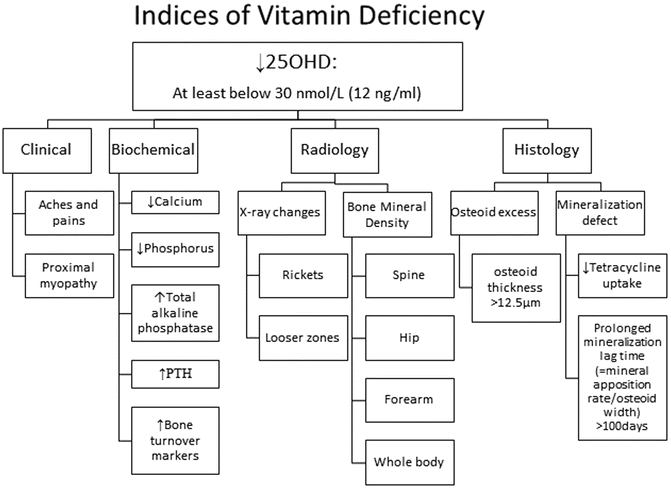
Fig. 23.2
25OHD = 25-hydroxyvitamin D; PTH = parathyroid hormone
If 25OHD is below 30 nmol/L (12 ng/ml), then the practitioner should encourage an augmented oral intake (see treatment section below), but does not necessarily need to embark on additional investigations. Much lower levels may be associated with clinical features including proximal myopathy and diffuse bone pain. Secondary biochemical indices include hypocalcaemia and hypophosphataemia. Although not calculated in clinical practice, the calcium–phosphorus ion product is a measure of the degree of deficiency that links directly with the consequence of a mineralization defect in bone. Another simple measure that is routinely available is serum total alkaline phosphatase; in the absence of liver disease it is a direct marker of bone disease.
Serum PTH should be measured as part of the assessment. Secondary hyperparathyroidism (SHPT) occurs in response to hypocalcaemia. This results in an increase in bone turnover as part of the effort to restore calcium homeostasis. In addition, 1α-hydroxylase activity is augmented such that 1,25(OH)2D levels may be elevated in vitamin D deficiency; this metabolite is not a measure of vitamin D status. Renal tubular effects of SHPT such as renal phosphorus wasting and renal bicarbonate wasting may hasten the onset of the mineralization defect in bone. Other factors influence PTH status such as calcium intake, renal function, age, ethnicity, body composition and geographic location [8]. There is no single threshold level of 25OHD that prevents secondary hyperparathyroidism [3, 8, 26].
An array of bone turnover markers is available for assessing bone status [27]. An increase in bone formation markers may reflect either an increase in bone remodelling activity due to SHPT or a defect in mineralization, or both. They are serum-based and should be collected in the fasting state (bone specific alkaline phosphatase, procollagen type I aminopropeptide and osteocalcin), whereas increased resorption markers only reflect SHPT. They include: (1) fasting serum-based tests such as beta-C-terminal cross-linking telopeptide of type I collagen (β-CTX), N-terminal cross-linking telopeptide of type I collagen (S-NTX), and tartrate-resistant acid phosphatase 5b (TRAP5b); and (2) either a timed-fasting urine or fasting second void urine or a 24-h urine collection for urinary NTX (U-NTX). Clinicians should obtain protocols from their laboratory service provider for instructions on specimen type required. It should be noted that reduced renal function may lead to reduced urinary excretion of β-CTX and a consequent increase in the apparent serum β-CTX concentration. Urinary markers of bone metabolism should be omitted in patients with renal insufficiency and a creatinine clearance of <20 ml/min [28]. In vitamin D deficiency both formation and resorption markers are increased, but in hypophosphataemic bone disease only formation markers are increased.
Specific radiographic changes occur late in the course of vitamin D deficiency. Rickets is a disease of the growing skeleton with radiographic changes being most pronounced at the growth plates in those bones that are growing fastest such as around the knee, the wrist especially the distal end of the ulna, the middle ribs, the proximal femur and the distal tibia. Initially the growth plate widens as a consequence of defective mineralization between epiphysis and metaphysis [29]. Then the metaphyseal surfaces become cupped and irregular. This is accompanied by splaying of the metaphyses and widening of the growth plates that accounts for the classical clinical signs of swelling at the wrists, knees and anterior ends of the ribs (rickety rosary). Bone deformities occur principally in lower extremities in weight-bearing bones resulting in knock knees, bow-legs, wind-swept legs [29, 30].
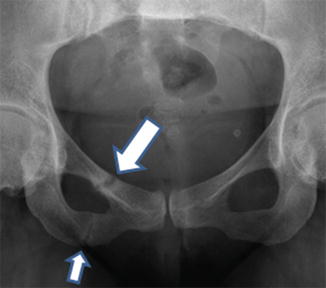
Insufficiency-type stress fractures in the setting of osteomalacia are referred to by the eponymous term, Looser zones [29]. They are often incorrectly called “pseudofractures”. It has been recommended for many years that this term is of no further value [31]. Looser zones are stress fractures. They are usually multiple in origin and are often symmetric in occurrence. They occur at typical sites in both weight-bearing bones (such as pubic rami, medial aspects of the femur and tibia, and metatarsal bones) and non-weight bearing bones (such as ribs, and medial border of the scapula). Appearances are characteristic in that the fracture appears as a broad rather than a narrow band, margins are parallel, marginal sclerosis is minimal, callus is usually present, but healing is delayed (Fig. 23.3). Typically, they only occur late as a manifestation of osteomalacia. Traditionally, they were considered to be pathognomic of osteomalacia, but rarely insufficiency-type stress fractures with appearances of Looser zones are described [29, 31].