Albuminuria—albumin/creatinine ratio
Serum creatinine
aeGFR-MDRD or CKD-EPI
Persistent albuminuria in the range of 30–299 mg/24 h (microalbuminuria) is considered the earliest stage of DN in type 1 diabetes (T1DM) and a marker for development of nephropathy in T2DM and for increased CVD risk [8].
The pathophysiological mechanisms in the development of DN are multifactorial. Hyperglycemia is related to structural and functional changes such as glomerular hyperfiltration, glomerular and tubular epithelial hypertrophy, and microalbuminuria, followed by the development of glomerular basement membrane (GBM) thickening, accumulation of mesangial matrix, evident proteinuria, and eventually glomerulosclerosis and ESRD. Nevertheless, intensive therapy to improve glycemic control is able to attenuate the development of nephropathy, as assessed by urinary albumin excretion (UAE), but not fully prevent it [9] (Fig. 36.1).
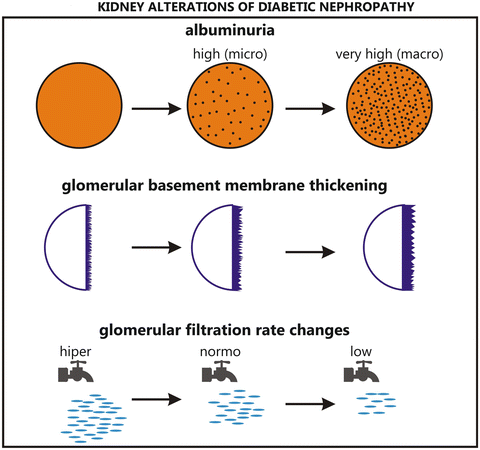

Fig. 36.1
Kidney alterations of diabetic nephropathy
Hemodynamic and metabolic pathways are involved in the development of DN. Hyperfiltration and hyperperfusion injuries occur very early in DN, and are glomerular hemodynamic changes related to the decrease of arteriolar resistance, more evident on the afferent side, which lead to a rise in glomerular capillary pressure. In addition to hyperglycemia, other factors, such as prostanoids, angiotensin II (ANGII), nitric oxide (NO), atrial natriuretic factor, growth hormone, glucagon, and insulin may be related to the increase in filtration and perfusion. Vascular endothelial growth factor (VEGF) and cytokines such as transforming growth factor–beta (TGFβ) increase NO production and mediate hyperfiltration. Glomerulosclerosis occurs as a result of high intraglomerular pressure, an increase in mesangial cell matrix production and GBM thickening [10, 11].
Hyperglycemia augments the oxidative stress and overproduction of reactive oxygen species (ROS) that stimulate protein kinase C (PKC) pathways, advanced glycosylation end-products (AGE) formation, TGFβ, and ANG-II [10].
Glucose transporter-1(GLUT-1) regulates the entry of glucose into the kidney cell and glucose activates the metabolic pathways. Nonenzymatic glycosylation of glucose produces AGE, activates PKC, and accelerates the polyol pathway; hemodynamic changes activate VEGF, TGFβ, interleukin-1 (IL-1), IL-6, IL-18, and tumor necrosis factor alpha (TNFα) and together increase albumin permeability in GBM and extracellular matrix accumulation, leading to elevated proteinuria, glomerulosclerosis, and tubulointerstitial fibrosis [11].
Pathologic abnormalities in the kidneys occur before the onset of microalbuminuria. The hallmark of DN is a nodular glomerulosclerosis, the Kimmelstiel-Wilson lesion [12], but less than one-third of diabetic patients with microalbuminuria have the typical glomerulopathy [13]. The earliest changes are an increase in the extracellular matrix and mesangeal cell hypertrophy. There is an increased deposition of type IV collagen in GBM, and the thickening may start as early as 1 year after the onset of T1DM, and later in glomerulosclerosis the deposition of collagen type 1 and III also occurs. Hyperglycemia impairs integrin expression and the structure and function of the podocytes, which are glomerular epithelial cells that cover the GBM. Hyperglycemia also reduces the number of podocytes, which is related to proteinuria, although this decrease is observed even in the absence of proteinuria and occurs before the development of glomerulosclerosis and tubulointerstitial damage [11] (Fig. 36.2).
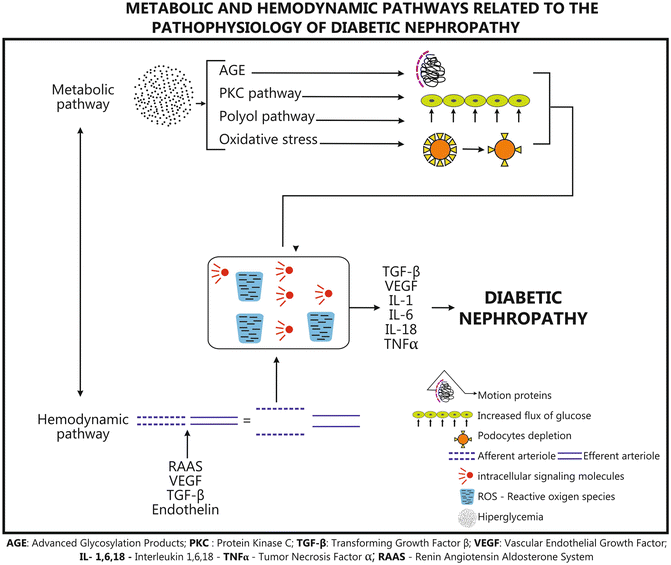

Fig. 36.2
Metabolic and hemodynamic pathways related to the pathophysiology of diabetic nephropathy
In view of the heterogeneity of kidney lesions and the complexity of the natural history of DN Tervaert et al., in 2010, defined four classes of DN according to the glomerular lesions found on electron microscopy that can be applied in both type 1 and type 2 diabetes [14]. In this classification class I is identified by an isolated GBM thickening (>430 nm in males over 9 years of age and >395 nm in females), with no evidence of mesangial expansion, increased mesangial matrix, or global glomerulosclerosis involving more than 50 % of the glomeruli, and glomeruli lesions then increase progressively to class IV, which is characterized by advanced diabetic sclerosis. (>50 % global glomerulosclerosis).
The “conventional” natural history of DN was defined in the 1980s, based on longitudinal studies of patients with type 1 and type 2 diabetes, and divided DN into five stages [15] as follows: stage 1 with a reversible glomerular hyperfiltration; stage 2 with normal GFR and normoalbuminuria; stage 3 GFR still normal but associated with microalbuminuria (5–10 years after diagnosis of DM); stage 4, in which proteinuria appears and may reach nephrotic range levels (after 10–20 years of diabetes progression); and stage 5, characterized by a GFR slope below 10 ml/min/year and CKD, leading to ESRD.
Information on the likelihood of passing from one stage to another in newly diagnosed patients was provided by the findings of the United Kingdom Prospective Diabetes Study (UKPDS) [16]. However, the study also emphasized that the risk of mortality increased in parallel with the worsening of renal disease. After 10 years of diagnosis 25 % of the patients with T2DM developed microalbuminuria and 5 % macroalbuminuria, and in the latter the death rate exceeded the rate of progression to an advanced stage of nephropathy [17].
The Diabetes Control and Complications Trial (DCCT) showed that less than 2 % of patients on intensive treatment developed renal failure after 30 years of diagnosis. The development of microalbuminuria in patients with T1DM usually begins 5–15 years after the onset of diabetes and increases progressively. Patients without proteinuria after 20–25 years have an approximately 1 % per year risk of developing clinical renal disease [9].
Nevertheless, another natural history of DN has been identified, particularly in type 1 and type 2 diabetic patients, although it is not clear why some patients develop the “classical” DN with significant proteinuria, while others have impaired renal function associated with very low levels of proteinuria that may persist until the ESRD [8, 15].
It would be useful to identify individuals, still normoalbuminuric, whose likelihood of progression to microalbuminuria is increased, but this is not yet possible. In addition to environmental influences, there is evidence in support of genetic susceptibility to microvascular complications of nephropathy in diabetic patients. Earlier investigations that focused on genetic mapping have generally yielded conflicting results, probably because, like other human diseases or syndromes, DN can develop from the interactions of several genes that in isolation would have no effect but which, when subtly altered, could predispose to DN [18].
Hence, it is important to enquire about the family history of DN and to screen periodically all diabetic patients. Microalbumin and serum creatinine (SCr) tests are valuable laboratory markers used to detect early signs of kidney damage [4]. A recent study that evaluated the risk stratification of kidney disease emphasized that both the urine microalbumin level and urine albumin/creatinine ratio tests are needed to fully assess kidney disease and its associated risks of death and progression to ESRD [19] (Table 36.2).
Table 36.2
Treatment targets of glycemia, blood pressure, dislipidemia
Glycemic control | HbA1C < 7, <6.5 | Caution with patients with advanced kidney disease and high-risk CVDa |
BPb control | <130 × 80 mmHg | Caution with patients with high-risk CVD |
LDLc | <100/dl, <70 mg/dl | Stage 5 of kidney disease: start statin only if specific CVD risk |
“Kidney Disease: Improving Global Outcomes” (KDIGO) conducted a meta-analysis of nine cohorts from the general population and another eight cohorts with a high risk for CKD, which confirmed that lower eGFR and higher albuminuria are risk factors for ESRD, acute kidney injury, and progressive CKD in both the general and high-risk populations, independently of each other and irrespective of cardiovascular risk factors [20].
The gold standard for GFR measurement is urinary clearance of an exogenous filtration marker, which is expensive and troublesome, and in addition to which it varies during the day. In clinical practice SCr is used to estimate GFR, applying the modification of diet in real disease (MDRD) and/or CKD epidemiology collaboration (CKD-EPI) equations [21], which use clinical variables as substitutes for unmeasured non-GFR determinants and provide more accurate estimates than SCr alone. Estimates of the CKD burden depend in part on the equation used to define the eGFR: when the more recent CKD-EPI equation is used, the prevalence of eGFR below 60 ml/min/1.73 m2 is lowered by a factor of 0.88 (6.9 versus 7.8 %), compared with the estimate from the older MDRD study equation [4].
In patients with T1DM the first screening is recommended at 5 years after the diagnosis [22], but it is suggested that patients with poor metabolic control be evaluated at the onset of puberty, which is an independent risk factor for microalbuminuria [23]. On the other hand, as about 7 % of the patients with type 2 diabetes will already have microalbuminuria at the time of diagnosis of diabetes, the screening must be started by then. If microalbuminuria is absent, the screening must be repeated annually for both type 1 and 2 diabetic patients [17].
In general, the Medical Societies recommend that an assessment of UAE be performed annually [24, 25], starting at the diagnosis of T2DM and 5 years after that for T1DM, in combination with a measurement of SCr in order to estimate GFR and determine the stage of CKD.
Kidney disease is classified in five stages [24] according to the GFR (ml/min per 1.73 m2 body surface area), considering kidney damage as abnormalities on pathologic, urine, blood, or imaging tests. Stage 1 is characterized by kidney damage with normal or increased GFR (≥90), stage 2 also by kidney damage associated with mildly decreased GFR (60–89), stage 3 by a moderately decreased GFR (30–59), stage 4 by a severely decreased GFR [15–29], and stage 5 as kidney failure defined as GFR below 15 or dialysis.
In February 2007, a consensus conference in the UK [26] approved the division of stage 3 CKD into stage 3A (eGFR 45–59) and stage 3B (eGFR 30–44) and added the suffix “p” to the GFR-based stage for patients with proteinuria (random urine protein:creatinine ratio >100 mg/mmol). These changes have been endorsed by the National Institute for Health and Clinical Excellence (NICE), the Scottish Intercollegiate Guidelines Network (SIGN) and the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI). Patients at stages 1–3 are considered to have early CKD.
The measurement of albuminuria may be performed by albumin-to-creatinine ratio (ACR) in a random spot collection, but also in 24-h or timed collections, which are less predictive and accurate [25]. If albuminuria is abnormal, the test should be confirmed by 2 or 3 samples within 3 or 6 months because albumin excretion may rise due to exercise within 24 h of sampling, infection, fever, congestive heart failure (CHF), marked hyperglycemia, hypercholesterolemia, and high blood pressure.
In the new nomenclature the term microalbuminuria (UAE—30–300 mg/24 h (20–200 μg/min) or ACR—30–300 mg/g) is replaced by “high albuminuria” and macroalbuminuria (UAE ≥300 mg/24 h (≥200 μg/min) or ACR ≥300 mg/g) by “very high albuminuria,” now recommended because the risk observed between urine ACR and CVD and between the former and renal disease is continuous; there is no specific threshold, and the risk is observed even in those with “high normal” range urine albumin excretion [27]. In addition, the term microalbuminuria does not reflect the amount of albumin, but small albumin molecules, and is becoming increasingly more confusing as a result of new evidence that urine may contain different immunoreactive moieties and fragments of albumin [28].
Differential Diagnosis
Very often clinicians tend to attribute proteinuria and renal impairment to DM, but that is not the only renal abnormality found in diabetics [29]. Other causes of CKD should be considered in patients that present with an absence of diabetic retinopathy, low or rapidly decreasing GFR, rapidly progressive proteinuria or nephrotic syndrome, refractory hypertension, presence of active urinary sediment, signs or symptoms of other systemic disease or a reduction in GFR of more than 30 % within 2–3 months after starting angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) [24]. Moreover in some patients the DN may be associated with other kidney diseases.
Nondiabetic renal disease (NDRD) includes a heterogeneous mixture of the following glomerular and nonglomerular conditions: [29]
1.
Glomerular disease other than diabetic nephropathy: immunoglobulin A nephropathy, focal and segmental glomerular sclerosis, microvascular complications of diabetes, membraneous glomerulonephritis, membranoproliferative glomerulonephritis, pauci immune, systemic lupus erythematosus, and others.
2.
Nonglomerular renal disease: macrovascular (renovascular), acute kidney injury (acute interstitial nephritis e.g. contrast nephropathy, sepsis, ACEI/ARBs/direct renin inhibitor (DRI) induced, and acute tubular necrosis e.g. sepsis, diuretic toxicity), electrolyte abnormality, urinary tract infection, etc.
Nevertheless, no consensus classification is available at the moment for kidney biopsy in a diabetic patient with any pathological condition.
Treatment—(Table 36.2)
Interventions that have been reported to be useful in preventing or retarding the progression of DN include the following: good glycemic and blood pressure control, treatment of hyperlipidemia, cessation of smoking, and restriction of protein intake. Patients who develop ESRD will require renal replacement therapy [30].
Blood pressure and glycemic control represent the major cornerstones for preventing and treating diabetic nephropathy [4, 9]. The DCCT reported that any decrease in hemoglobin A1C (HbA1C) was strongly associated with a reduction in the risk of developing microalbuminuria and progression to overt nephropathy [9], and UKPDS clearly demonstrated a role for intensified glycemic control in subjects newly diagnosed with T2DM, in whom treatment led to a fall in HbA1C from 7.9 to 7.0 % [31].
To reduce the risk or slow the progression of nephropathy the American Diabetes Association (ADA) recommends the optimization of glucose and control of blood pressure. Recently, the ADVANCE study demonstrated that the decrease in HbA1C to a mean of 6.5 % was associated with a further reduction in renal events, as assessed by the development and progression of microalbuminuria [32]. However, the findings of the ACCORD study [33] led to controversy regarding the appropriate HbA1C target for reducing macrovascular disease.
The major risk of reaching HbA1C levels below 7.0 % is the increased likelihood of developing hypoglycemia. For people with decreased kidney function (CKD stages 3–5), hypoglycemia is a major concern because it impairs the clearance of insulin and a number of oral agents used to treat diabetes, as well as reducing kidney gluconeogenesis [24]. Drug adjustments must be made to prevent or, at least, reduce the risk of hypoglycemia.
Sulfonylureas in general have predominantly renal elimination and are not recommended for patients with creatinine clearance (CrCl) below 50 ml/min, except for glypizide, which has hepatic elimination of inactive metabolites and should be interrupted when CrCl falls below 30 ml/min. Malnutrition, acute illness, liver disease, and alcoholism are risk factors for hypoglycemia. Meglitinides are oxidized by the liver but still entail a risk of hypoglycemia because active metabolites may accumulate in renal dysfunction, repaglinide being the one that accumulates the smallest amount of metabolites. Metformin is eliminated unchanged by the kidneys; NKF-KDOQI contraindicated its use with a serum creatinine over 1.5 mg/dl in males and 1.4 mg/dl in women due to the risk of lactic acidosis, although NICE recommends that it should be used with care for patients with an eGFR below 45 ml/min/1.73 m2 and discontinued if the eGFR falls below 30 ml/min/1.73 m2. Acarbose is not recommended if CrCl is below 25 ml/min, and miglitol produces renal elimination, but as there are no studies in patients with kidney disease, FDA do not recommend either of them if serum creatinine is ≥2 mg/dl. The risk of side effects when using thiazolidinediones increases with renal disease [24, 34].
Exenatide and its formulation with extended release are eliminated by renal filtration and need no adjustment with CrCl above 50 ml/min. Increases in the dosage from 5 to 10 μg should be applied with care if CrCl is 30–50 ml/min and, according to FDA, when CrCl is below 30 ml/min it should be stopped. Liruglutide should be used with care when CrCl is below 60 ml/min, and when below 30 ml/min its side effects increase, but experience of its use is still limited in CKD. The dipeptidyl peptidase-4 (DPP4) inhibitor agents need no adjustment if CrCl ≥50 ml/min; sitagliptine should be reduced to 50 mg/d if it is 30–50 ml/min and to 25 mg if <30 and saxagliptine to 2.5 mg if <50 ml/min. Linagliptine is fecally eliminated unchanged, so it may be safely used in patients with CKD. Colesevalem and bromocriptin need no adjustments. As up to 50 % of insulin is eliminated by the kidney, it is recommended that it be reduced by 25 % when CrCl is 10–50 ml/min and by 50 % if it falls below 10 ml/min [24, 34].
In addition to the importance of glycemic control, it has been shown that a more aggressive BP reduction reduces the progression of DN. The mechanism of hypertension in DN is complex and not fully understood, being related to excessive sodium retention, activation of the sympathetic nervous system (SNS) and the renin–angiotensin–aldosterone system (RAAS), augmented oxidative stress, and endothelial cell dysfunction (ECD) [35].
The UKPDS provided strong evidence that control of BP can slow the development of nephropathy [36]. Treatment using angiotensin-converting enzyme inhibitors (ACEi) retards the progression from micro- to macro-albuminuria and can slow the reduction of the GFR in patients with macroalbuminuria [37, 38]. In T2DM with hypertension and normoalbuminuria, renin-angiotensin system (RAS) inhibition has been shown to delay the onset of microalbuminuria [39, 40]. The evidences suggest that ACE inhibitors [41] have renoprotective actions in addition to their antihypertensive effects for primary prevention [42].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






