Fig. 7.1
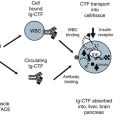
Urinary trypsin inhibitors are release from non-inhibitory pro-inhibitors. (Step 1) Serine proteases of the trypsin family (Elastase, Cathepsin, Tryptase, Trypsin, Kallikrein, Thrombin, Plasmin and Factors VII & X) are increased during inflammation by innate immune cells (e.g. White Blood Cells; Neutrophils, Monocytes, Eosinophils, Natural killer cells, Macrophages, Mast cells) and affected tissues (Epithelial, Endothelial, Smooth muscle, Fibroblast, Platelets and Neoplastic). Step 2) These proteases produce inflammatory response of vascular dilation, coagulation, and leukocyte infiltration with destruction of pathogens. Step 3) Serine proteases (mainly elastase) liberate plasma bikunin (Bik) the primary uTi from pro-inhibitor, inter-α-inhibitor (I-α-I), activating the inhibitory response. Bik is rapidly excreted into urine. Step 4) Bik inhibits serine proteases as an anti-inflammatory response controlling remodeling, smooth muscle contraction, fluid/electrolyte balance and protecting tissue from leukocyte damage. Step 5) Bik signals cells to reduce proliferation, inflammation cytokine mediator release, growth factor activation, uPA activation and intra-cellular Ca+ slowing cell growth. Step 6) Bik is rapidly metabolized and excreted into urine avoiding a prolonged suppression of inflammatory system. Uristatin (Uri) is a primary form in urine and lacks the O-linked glycoside
Urinary tract infections are the primary cause of glomerular nephritis as immune cells responding to infection damage tissue and cause a pro-inflammatory response (Hooton and Stamm 1997; Barnett and Stephens 1997; Saint et al. 1991). The kidney model shown in Figs. 7.2, 7.3 and 7.4 can be used to explain the impact of inflammation and uTi on the kidney. The kidney components and blood and urine flow are shown in Fig. 7.2. Pro- and anti-inflammatory processes involved in kidney damage and regeneration can be explained by this model. In normal kidneys, only low molecular weight (LMW) proteins (<30 kDa) pass the basement membrane of the glomerular capillary into the distal collecting tube where proximal tubular cells re-absorb this protein back into the blood leaving no proteinuria (Fig. 7.3). In abnormal kidneys, where the basement membrane has been damaged, large proteins (> = 30 kDa) pass the basement membrane of the glomerular capillary into the distal collecting tube and proximal tubular cells can fail to re-absorb high amounts of protein into the circulation leaving gross proteinuria and loss of kidney function (decline of glomerular filtration rate (GFR)) (Fig. 7.4).
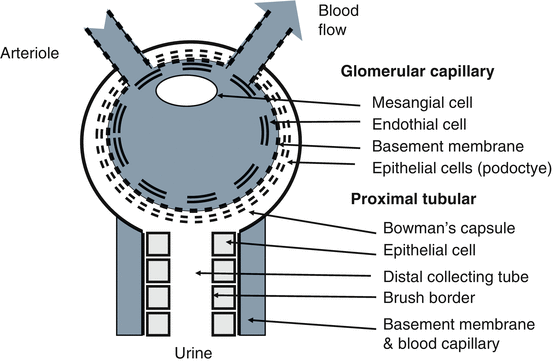
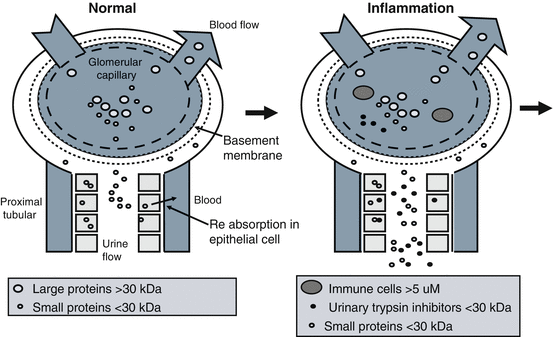
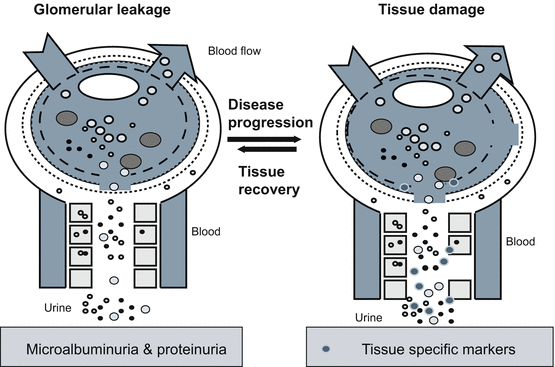

Fig. 7.2
Kidney model. The kidney model is to explain the impact uristatin on kidney during urinary tract infection and inflammation. Key kidney components for glomerular capillary and proximal tubular are shown

Fig. 7.3
Early kidney inflammation. In normal kidney, only the small proteins pass the basement membrane of the glomerular capillary into the distal collecting tube and proximal tubular cells re-absorb this this protein into the blood leaving no proteinuria. In kidney where the basement membrane has been damaged, large proteins pass the basement membrane of the glomerular capillary into the distal collecting and tube proximal tubular cells fail to re-absorb this protein into the blood leaving proteinuria. Prior to iamass immune cells such as white blood cells response to infection and migrate to inflamed tissue. The WBCs cause uTi to be released into the urine. The uTi correlates low molecular weight proteinuria presumed to be due to shut down tubular re-absorption. Inflamed tissue can also release serine proteases that are able to generate uTi from its pro-inhibitor

Fig. 7.4
Tissue recovery or disease progression. White blood cells such as Neutrophils and Macrophages lead to lesions in the basement membrane. Damage continues without a strong anti-inflammatory response by urinary trypsin inhibitors (uTi) to shut down the immune mediated apoptosis. Microalbuminuria is first detectable before the loss of renal function or of change in glomerular filtration by blood markers. As tissue damages progresses and the glomerular leakage of high molecular weight protein occurs and increases the amount of protein spilled into urine. Regeneration of renal cells such as podocytes is possible at early stages and recovery of kidney possible. In absence of regeneration, complement form and the disease will progress
As kidney tissue becomes inflamed, white blood cells (WBC) migrate into the interstitial space to fight infection. Neutrophilic elastase is released from these WBC. Elastase proteolysis of pro-inhibitors give rise to uTi. Interleukin-α-inhibitor (I-α-I) and pre-interleukin-α-inhibitor (P-α-I) are the primary pro-inhibitor forms (Fig. 7.1). More than 98 % of the uTi in blood is bound to these pro-inhibitor forms and is inactive from inhibition of serine proteases. Bikunin (Bik) and Uristatin (Uri) are two key forms of uTi. Uristatins (Uri) are small derivatives of Bikunin lacking the O-linked glycoconjugates.
The uTi released during infections has a potent anti-inflammatory response (Fig. 7.1). The presence of uTi correlates with febrile proteinuria and elevated white blood cells (WBC) during urinary tract and respiratory infections (Pugia et al. 2002, 2004). Low molecular weight (LMW) urinary proteins also increase during febrile proteinuria. These LMW proteins are not reabsorbed by the proximal tubular. Microalbuminuria (30–300 mg/L of albumin) is also detected during fever (Pugia et al. 2002). In cell models uTi has been shown to shut down the proteases need for tubular re-absorption and potentially facilitating this excretion of LMW proteinuria of during fever (See Chap. 10). Febrile proteinuria is gone once WBC returns to normal and fever is gone. This temporary condition does not cause loss of renal function or change glomerular filtration rates.
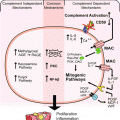
This potent anti-inflammatory agent has been used as an anti-shock treatment and to protect tissues by suppressing vascular dilation, coagulation, and smooth muscle contraction during wound healing (Pugia and Lott 2005). Studies have shown uTi to protect kidney tissues from immune mediated apoptosis in animal models during glomerulonephritis (Koizumi et al. 2000; Takahashi et al. 2001; Nakakuki et al. 1996). There are also uTi released in response to chronically inflamed tissue. The benefits of these protective effects of uTi have been shown during disseminated intravascular coagulation, ischemia-reperfusion injury, endothelial injury and acute renal failure (Eguchi 2003; Murakami 1988; Yamaguchi et al. 2000; Nakatani et al. 2001; Onbe et al. 1991). uTi has also been shown to reduce release of growth factors and cytokines, inhibit growth factor activation, and stop protease-activated receptor (PAR) signaling in cell models (Fig. 7.1) (Pugia et al. 2007b).
Persistent white blood cells (WBC) will mediate cell death in tissues and lead to glomerular nephritis causing lesions in basement membrane (Cochrane et al. 1965). Glomerular nephritis is characterized by immune complex deposition in kidney and is observed with hematuria and proteinuria (McCluskey 1970; Hatano 1984; Couser 1998). Damage can occur through an immune mediated apoptosis process (Bonventre and Weinberg 2003). Untreated glomerular nephritis progresses to chronic kidney disease (CKD). The inflammatory response will transition from the innate immune response to complement and auto immune responses (McCluskey 1970; Pickering et al. 2013). Chronically inflamed tissue will develop glomerular sclerosis with glomerular C3 fragment deposition (Pickering et al. 2013; Cameron 2003). As glomerular sclerosis develops, the kidney tissue fails to regenerate to normal state. These chronically inflamed tissues promote proliferation by release of serine proteases to activate protease-activated receptors (PAR) (Vesey et al. 2013; Coelho et al. 2003; Tull et al. 2012). The key proteases causing this apoptosis and proliferation are known to be inhibited by uTi (Pugia et al. 2007b). In the absence of uTi protecting normal tissue, the damage increases and proliferation occurs leading to proteinuria (>300 mg/L) and decreased glomerular filtration rate (GFR) (Fig. 7.4).
The kidneys can recover if protected from immune cells and allowed to regenerate normally (Yoshida and Honma 2014). The anti-inflammatory response appears to be central to the ability of the kidney to regenerate and naturally reduce these complications. Here, uTi is an ideal candidate for assessment of immune cell mediated kidney damage in chronically inflamed kidney tissue. However the role of uTi in progression from a healthy kidney to damaged tissue is not completely known. uTi offers value for identifying glomerular nephritis patients under anti-inflammatory stress.
Urinary trypsin inhibitors (uTi) in a urine specimen can be measured by inhibition of trypsin in a dipstick method based on a newly found enzyme substrate (Pugia et al. 2002, 2004; Jortani et al. 2004). The dipstick is a urine test alternative to a blood C-reactive protein (CRP), a complete blood count (CBC) or sedimentation rate in patients with upper respiratory infections and other infections owing to bacteria and surpassed these measures in patients with urinary tract infections (UTI). Clinical use of inhibition assays for urinary trypsin inhibitors were also demonstrated useful as acute phase reactant and for renal disease by other groups (Kuwajima et al. 1989, 1992; Takemura et al. 1994; Mizon et al. 2002).
Immunoassays for uTi are typically unable to distinguish among the various forms of urinary trypsin inhibitors from pro-inhibitors (I-α-I and P-α-I) (Pugia et al. 2002; Trefz et al. 1991). The cross-reactivity negates blood specimens where I-α-I and P-α-I are present in healthy patients. Western blot analysis of urine identified uristatin and bikunin were key forms of uTi (Pugia et al. 2002). A monoclonal antibody (IATI5) produced against I-α-I also recognized P-α-I, AMBK and bikunin (Trefz et al. 1991). Others showed monoclonal antibodies raised against AMBK were reactive to uristatin, AMBK, and bikunin (Bik) (Kobayashi et al. 2000).
New monoclonal immunoassays were developed that do not show an interfering cross reactivity to pro-inhibitors in blood and urine (Pugia et al. 2007a). The antibodies uncovered an impact of glycoconjugation, measured certain glycoforms could detect uristatin Uri and bikunin. The primary uTi remaining in blood is Bik with two Kunitz inhibitor domains and O-linked and N-linked polysaccharides. In urine, Uri are derivatives of Bik, lacking the O-linked glycoconjugates chondroitin sulfate chains are often found along with Bik. The urine immunoassay has been used to measure both Bik and Uri in urine.
The immunoassay detects forms that are more common in kidney disease, thus we explored the application of this immunoassays for kidney diseases by making use of an extensive clinical data and sample bank from previous studies (n = 6294 patients). It was possible that these new uTi assays could predict glomerular nephritis progressing to chronic kidney disease as an indicator of damaging inflammation and infection. The goal was to test the role of Uri and Bik and to better understand the involvement in the stages of nephritis.
7.2 Methods
7.2.1 Immunoassay for Clinical Analysis
Patients’ urine specimens were tested using a competitive ELISA method previously described with new antibodies (See Table 7.1) (Pugia et al. 2007a). The immunoassay measures both Uri and Bik but does not cross-react with IαI or pαI. Calibration of the immunoassay used Uri isolated from human patients with chronic renal failure (SciPac, Sittingbourne, Kent, UK, product code P392-1). All lots were stable at −20 °C to −70 °C, either in solution or as lyophilized solids and previously described (Pugia et al. 2007a).
Table 7.1
Antibodies for Uristatin and Bikunin
Antibody | Raised against | Clone/lot |
|---|---|---|
Uristatin/Bikunin N-link glycan binding | Human Uristatin | ATCC 420-5D11.5G8.1E4 PTA-7744 |
Bikunin/Uristatin tyrpsin inhibitory peptide domain binding | Human Uristatin | ATCC 421-3G5.4C5.3B6 PTA-7746 ATCC 421-5G8.1AB.5C1 PTA-7745 |
mAb for Uristatin/Bikunin conjugated to ALP | Human Uristatin | ATCC 421-3G5.4C5.3B6 |
pAb for Uristatin/Bikunin conjugated to ALP | Urinary trypsin inhibitors | Rabbit polyconal |
The ELISA Method for Uri is initiated by coating each well with 0.1 ug/well Uri in TBS (Tris buffered saline) overnight at 4 °C followed by washing five times with 300 μL of TBS per well (Elx 405 Auto Plate Washer, Biotek Instruments, Winooski, VT). The plate is blocked with 300 μL of SuperBlocker® per well, incubated for 60 min at 37.5 °C (JitterBug Microplate Shaker, Beokel Scientific, Feasterville, PA), and finally washed the plates five times with 300 μL of TBS-with 05 % Tween-20 per well. Calibrators made with Uri are diluted with uristatin antibody conjugated to alkaline phoshatase (ALP-Mab) conjugates in TBS at 0.084 μg/L (mg/L). A sample is made by diluting urine with the required a 100-fold dilution of ALP-Mab. Diluted calibrator or specimen in 100 μl volumes are loaded into the coated wells, incubated at 35 °C for 1 h, and washed five times with 300 μLTBS-Tween per well. The antibody binding is measured at 37 °C over 30 min after addition of PNPP 100 μL/well (100 ng/mL) (1-Step™ solution, Pierce), by reading the change in 410 nm absorbance on a FLx 800 microplate Reader (Biotek Instruments). (See on-line supplement for ELISA Method for Uristatin for additional details at www.extras.springer.com/2015/978-3-319-21926-4). A uristatin positive is considerd as ≥12 mg/L.
Some patients’ urine specimens were also tested using a western blot method with rabbit polyclonal antibody conjugated to ALP A described (Table 7.1) (Pugia et al. 2004). The antibody cross-reacted with Uri, Bik, P-a-I, I-a-I, and AMBP. Urine specimens from diabetics were characterized using a commercial pre-cast SDS-PAGE gel system (BioRad). In brief, urine from diabetic patients was concentrated to about 20 μg total protein and loaded onto the gel along with molecular-weight markers and Bik standards. The SDS-PAGE gel was ran in 25 mM Tris, pH 8.3, 192 mM glycine, and 0.1 % SDS buffer at 110 V in for 1–1.5 h until complete. The gel was transferred to the blotting cassette using filter paper. The gel soaked in transfer buffer and proteins transfers to nitrocellulose paper at 30 V in a 4 °C chamber overnight. The membrane was washed in TBS buffer plus 0.1 % Tween-20, blocked with 5 % BSA (Sigma A3059) and reacted rabbit anti-uristatin antiserum at a dilution of 1:250,000 at 4 °C overnight with gentle agitation. The membrane was incubated with the alkaline phosphate substrate and color developed and recorded. (See on-line supplement for Western Blot Methods for additional details at www.extras.springer.com/2015/978-3-319-21926-4)
7.2.2 Sample and Data Bank
All clinical and diagnostic data was collated into one database from four previous studies. The combined population (n = 6294) was intended to mimic the natural prevalence of renal disease (3 %), urinary tract infection (3 %), nephritis (15 %) and systemic infection (2 %) expected in a general population without exclusion or selection criteria. Clinical samples stored at −70 °C were thawed and used once for additional testing for uTi by the Uri ELISA immunoassay methods. Uri values by ELISA of <12 mg/L was considered normal. Test strips for urinary tract infection had been tested in original studies. Here a Uri by dipstick of < = 7.5 mg uristatin/g creatinine or <12.5 mg/L Uri was considered normal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree