Cytokines that Share the Common Cytokine Receptor γChain (Interleukin-2, Interleukin-4, Interleukin-7, Interleukin-9, Interleukin-15, and Interleukin-21)
The receptors for six different immunologically important cytokines, IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21, share the common cytokine receptor γ chain, γ
c (CD132).
11,30,36,37,38,39,40,41,42,43,44,45 These cytokines are all short-chain four α-helical bundle cytokines; basic features of these cytokines are summarized in
Table 25.3. The properties of these cytokines and their distinctive receptor chains are summarized in the following text, followed by a discussion of the discovery that they share a common receptor component and the implications thereof.
IL-2 is important not only for its function but also historically, as it was the first type I cytokine that was cloned,
46 the first type I cytokine for which a receptor component was cloned,
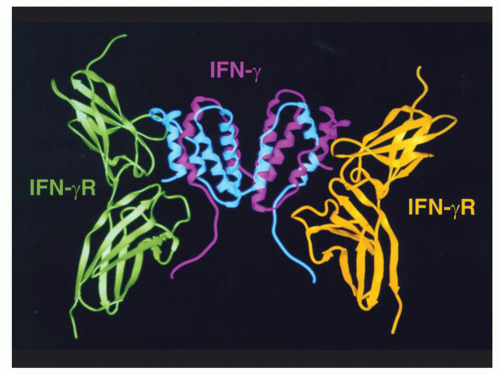
47,48 and the first short-chain type I cytokine whose receptor structure was solved.
23 Many general principles have derived from studies of this cytokine, including its being the first cytokine that was demonstrated to act in a growth factor-like fashion through specific high-affinity receptors, analogous to the growth factors being studied by endocrinologists and biochemists.
49,50Mature IL-2 is a 133 amino acid long peptide that can act as a major T-cell growth factor, in keeping with its original discovery as a T-cell growth factor.
51 Although IL-2 is not produced by resting T cells, it is rapidly and potently induced following antigen encounter with resting CD4+ T cells, and transcription and synthesis of IL-2 are often used as indicators of T-cell receptor (TCR)-mediated cellular activation. Although the antigen determines the specificity of the T-cell immune response, the interaction of IL-2 with high-affinity IL-2 receptors regulates the magnitude and duration of the subsequent response, based on the amount of IL-2 produced, the levels of high-affinity receptors expressed, and the duration of IL-2 production and receptor expression. IL-2 can act in either an autocrine or paracrine fashion, depending on whether the producing cell is also the responding cell or whether the responding cell is a nonproducing cell. The gene encoding IL-2 is located on chromosome 4,
52 and like many other helical cytokines, its gene consists of four exons.
7 IL-2 binds to three different classes of receptors. These are formed by different combinations of three different chains, IL-2Rα,
47,48,53 IL-2Rβ,
54,55,56,57 and a protein initially called IL-2Rγ
36 but now known as the common cytokine receptor γ chain, γ
c.
11,30,37 These different classes of IL-2 receptors are discussed in the following text.
In addition to its being a T-cell growth factor, IL-2 has other important actions as well (see
Table 25.3).
29 For example, it can increase Ig synthesis and J chain transcription in B cells,
50,58,59 potently augment the cytolytic activity of
natural killer (NK) cells,
60,61,62 induce the cytolytic activity of lymphokine activated killer cells, promote the elimination of autoreactive cells in a process known as antigen-induced (or activation-induced) cell death,
29,50,63 and promote the differentiation of regulatory T (T
reg) cells,
64,65 cells that suppress inappropriate responses and are important for immunologic tolerance. Low-dose IL-2 is sufficient to promote T
reg-cell survival, and thereby, for example, can protect mice from developing autoimmune diabetes.
66 Interestingly, IL-2 can also prime CD8+ T cells during a primary response to undergo enhanced proliferation in vivo during a secondary response,
67,68 and autocrine IL-2 is believed to be required for secondary expansion of CD8+ memory T cells.
69 There appears to be a complex interplay between IL-2 and inflammatory signals to regulate effector and memory cytolytic T-lymphocytes generation in lymphocytic choriomeningitis virus infection, with persistent IL-2 promoting effector rather than memory cytotoxic T-lymphocyte development.
70,71 In
Listeria monocytogenesis infection, Th1 effector memory cells highly express the transcription factor T-Bet and IL-2Rα, and IL-2Rα appears to be critical for the development of these cells.
72 Presumably because of its critical role in T
reg development, the absence of IL-2, IL-2Rα, or IL-2Rβ leads to autoimmunity. Interestingly, the production of IL-2 by mast cells has been reported to contribute to the suppression of chronic allergic dermatitis by its increasing the relative number of T
reg cells at the site of inflammation.
73IL-2 is also important in inducing or inhibiting Th differentiation. It is required for efficient Th1 74 and Th2 differentiation,
75,76,77,78 which are populations of T helper cells first described based on patterns of cytokine production by mouse T cells.
79 This theme was then extended to human cells as well,
80,81,82,83,84 although there are some variations in humans and mice in terms of the degree of how tightly restricted cytokine expression is. IFNγ is the cytokine most reliably produced by Th1 cells; IL-2 is also produced by these cells, although without as rigorous an association. In contrast, IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13 are produced by Th2 cells. In both species, certain type I cytokines (eg, IL-3 and GM-CSF) are produced by both Th1 and Th2 cells. IL-12 and the transcription factors signal transducer and activator of transcription (STAT)4 and T-Bet are the major transcription factors promoting Th1 differentiation, whereas IL-4 and the transcription factor STAT6 drive Th2 differentiation. For Th1 cells, IL-2 acts by STAT5-dependent induction and expression of IL-12Rβ1/IL-12Rβ2 and T-Bet,
74 whereas for Th2 differentiation, IL-2 via STAT5 promotes IL-4
75,76 and IL-4Rα
77 expression. In addition, IL-2 also affects chromatin remodeling at the
Ifng locus during Th1 differentiation.
85 IL-2 also inhibits Th17 differentiation,
86 in part via downregulation of IL-6Rα/gp130 expression
74 and by inducing T-bet,
74,87 which prevents Runx1-mediated activation of RORγt, a transcription factor that drives Th17 differentiation.
87Like IL-2, IL-4 is produced primarily by activated CD4+ T cells.
88,89 IL-4 is also produced by CD4+NK1.1+ “natural” T cells, denoted as natural killer T (NKT) cells,
90 and by mast cells and basophils.
88 IL-4 is the major B-cell growth factor, and it promotes Ig class switch, enhancing the production and secretion of mouse IgG1 (human IgG4) and being essential for the production of IgE.
88 IL-4 is involved in the physiologic response to parasites, including helminths, and for allergen sensitization. IL-4 induces expression of class II major histocompatibility complex molecules and increases cell surface expression of the CD23 (the low-affinity IgE receptor) on B cells. In addition to its actions on B cells, IL-4 can also act as a T-cell growth factor, inducing proliferation in both human and mouse T cells, and is critical for normal differentiation of Th2 cells.
89 Moreover, when IL-4 and transforming growth factor (TGF)-β are combined, cells that produce IL-9 (Th9) cells are induced (see following discussion). When combined with phorbol 2-myristate 3-acetate, IL-4 is also a potent comitogen for thymocytes. Importantly, IL-4 can inhibit certain responses of cells to IL-2.
91 Moreover, IL-4 can exert actions on macrophages, hematopoietic precursor cells, stromal cells, and fibroblasts.
92 The gene encoding IL-4 is located on human chromosome 5 (5q23.3-31.2) and mouse chromosome 11,
92,93 in the same region as IL-3, IL-5, IL-13, and GM-CSF. The type I IL-4 receptor is expressed on T cells and other hematopoietic cells consists of the 140 kDa IL-4Rα protein
88,94,95,96 and γ
c.
38,39 Expression of IL-4Rα tends to be quite low, and cells that potently respond to IL-4 often express only a few hundred receptors per cell. In addition to the type I IL-4 receptor, an alternate form of the receptor (the type II IL-4 receptor), containing IL-4Rα and IL-13Rα1, although not expressed on mature T cells, is expressed on many other cell types and can transduce IL-4 signals into these cells. For example, IL-13Rα1 is expressed on neonatal Th1 cells and the type II IL-4 receptor has been implicated as mediating apoptosis of these cells.
97IL-7 is not produced by lymphocytes but instead is a 152 amino acid long cytokine that is produced by stromal cells and certain other cells.
98,99,100 Based on the analysis of patients with X-linked severe combined immunodeficiency (SCID), JAK3-deficient SCID, and IL-7Rα-deficient SCID, IL-7 receptor-dependent signaling is essential for T-cell development in humans (discussed subsequently). The major role of IL-7 is to enhance thymocyte survival, growth, and differentiation
101,102,103,104,105 as well as low-affinity peptide-induced proliferation, and thus it promotes homeostatic proliferation of naive and memory CD8+ T cells.
100,105,106,107 Additionally, IL-7 can regulate the homeostasis of CD4+ memory T cells
108,109 and also can stimulate the growth of mature T cells.
30,105,110,111 Although IL-7, as noted previously, is believed to primarily be a stromal factor, IL-7 has also been noted to be produced by the liver and kidney but not spleen or lymph nodes after toll-like receptor (TLR) signaling. Although basal liver production is low, after TLR signaling, hepatic IL-7 can promote CD4 and CD8 T-cell survival and promotes antigen-specific T-cell responses.
112 In addition, in the mouse, IL-7 is vital for the growth of mouse pre-B cells,
96,98,103,104,111 and transient IL-7 signaling can inhibit Ig heavy chain gene rearrangements.
113 IL-7 via STAT5 has also been shown to inhibit Ig-κ recombination in pro-B cells.
114 In contrast to its requirement for B-cell development in the mouse, there is normal B-cell development in patients with defective IL-7 signaling, as is found in patients with X-linked SCID, JAK3-deficient
SCID, and IL-7Rα-deficient SCID (see following discussion). Thus, human B cells can develop normally in the absence of IL-7 responsiveness, demonstrating that in humans, IL-7 is not vital for the growth of human pre-B cells,
30,115 and it remains unknown whether IL-7 plays important roles in human B-cell biology. Interestingly, IL-7 also acts on DCs, and this appears to limit the homeostatic proliferation of T cells under lymphopenic conditions.
100,116 Clinically, IL-7 has been of interest in terms of its ability to induce T-cell proliferation and to thereby increase T-cell numbers, with a greater effect on CD8+ than on CD4+ T cells, as well as to augment the diversity of the TCR repertoire
117; IL-7 also can increase antigen-specific responses following vaccination
100 and can promote antiviral immunity, apparently in part due to its induction of IL-22 and repression of SOCS3.
118 The gene encoding IL-7 is located on human chromosome 8q12 to 8q13
119 and mouse chromosome 3. The functional IL-7 receptor contains the 75 kDa IL-7Rα
120 and γ
c.
37,40 Interestingly, signaling via the TCR, IL-2 or IL-7 can downregulate IL-7Rα expression,
121,122 with PI 3-kinase/AKT and GFI1B being implicated in its downregulation in T cells.
122,123,124 The downregulation of IL-7Rα not only can both decrease responsiveness of cells but can also increase the availability of the cytokine for other cells that are poised to respond.
11 Induction of IL-7Rα requires PU.1 in B cells
125 and another Ets family protein, GABP, in T cells.
123IL-9 was originally described as a mouse T-cell growth factor
126 that is produced by activated T cells and can support the growth of T-helper clones but not of cytolytic clones.
127 In contrast to IL-2, its production is delayed, suggesting its involvement in later, perhaps secondary signals. In the mouse, IL-9 can exert proliferative effects on erythroid progenitors, B cells, B-1 cells, mast cells, and fetal thymocytes. IL-9 is identical to mast cell growth-enhancing activity, a factor present in conditioned medium from splenocytes,
128 and synergizes with IL-3 for maximal mast cell proliferation. IL-9 is highly expressed in the lung of patients with asthma,
129 and overexpression leads to airway inflammation and Th2 cytokine production.
130 Interestingly, IL-9 is substantially made in the lung by innate lymphoid cells, and in these cells neutralizing antibodies to IL-9 lowered expression of IL-13 and IL-5, supporting a link between IL-9 and the regulation of the Th2 response.
131 Consistent with the action of IL-9 on thymocytes in vitro, IL-9 transgenic mice develop thymic lymphomas, and IL-9 is a major antiapoptotic factor for such tumors.
132 Nevertheless, IL-9 knockout mice have normal T-cell development
133; instead, they exhibit a defect in pulmonary goblet cell hyperplasia and mastocytosis following challenge with
Schistosoma mansoni eggs, a synchronous pulmonary granuloma formation model. However, there was no defect in eosinophilia or granuloma formation.
133 Mice expressing an IL-9 transgene in the lung exhibit airway inflammation and bronchial hyperresponsiveness; nevertheless, IL-9-/- mice exhibit normal eosinophilia and airway hyperreactivity in an ovalbumin-induced inflammatory model.
134,135,136 Thus, although IL-9 can contribute to allergic/pulmonary responses, there are compensatory cytokines that substitute for IL-9 in at least certain settings. IL-9 can be produced by the Th9 populations of cells, which can be induced by IL-4 plus TGF-β in a fashion that is dependent on PU.1 and interferon regulatory factor (IRF)-4.
137 Interestingly, the addition of IL-25 further enhances the production of IL-9.
138 IL-9-producing cells are typically IL-9+IL-10+Foxp3-effector T cells and can be derived from Th2 cells.
139,140 Overall, IL-9 is believed to play a key role in allergic responses; additionally, given the ability of both Th17 and T
reg cells to produce IL-9 during autoimmunity and transplantation, it will be interesting to further clarify the role of IL-9 in autoimmunity.
141 Currently, there is some confusion, as one study showed that antibody blockade of IL-9 or IL-9Rα blocks experimental autoimmune encephalomyelitis (EAE) disease progression,
142 whereas another study showed that IL-9Rα knockout mice develop more severe EAE.
138 While mouse IL-9 is active on human cells, human IL-9 is not biologically active on mouse cells (the opposite situation from that for IL-2). Human IL-9 is located on chromosome 5q31 to 5q35,
143 which is also the location for the genes encoding IL-3, IL-4, IL-5, IL-13, and GM-CSF. In contrast, mouse IL-9 is “isolated” on chromosome 13, while IL-3, IL-4, IL-5, IL-13, and GM-CSF are clustered on chromosome 11. IL-9 binds to the 64 kDa IL-9Rα binding protein, which is similar in size to γ
c,
144 and the functional IL-9 receptor consists of IL-9Rα plus γ
c.
30,41,42IL-15 was identified as a T-cell growth factor that also unexpectedly was expressed in the supernatant of a human T-lymphotropic virus type I (HTLV-I)-transformed T-cell line.
145,146 Although IL-15 messenger ribonucleic acid (RNA) is produced by a range of nonlymphocytic cell types, it is difficult to detect physiologic levels of IL-15 protein.
147 Its main site of synthesis appears to be DCs and monocytes, and unlike IL-2, IL-15 is not produced by activated T cells.
148 IL-15 receptors are widely expressed; although IL-15 is perhaps most important for the development of NK cells
149,150,151 and CD8+ memory T cells,
150,151 it also has both paracrine and autocrine actions on DCs, including promoting the survival of these cells.
11 Interestingly, it also regulates the TCR repertoire of γδ intraepithelial lymphocytes
152 and cross talk between different types of DCs.
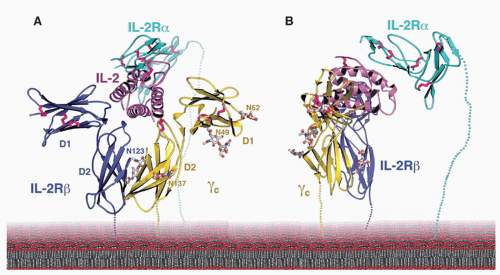
153 The receptor for IL-15 on T cells contains IL-2Rβ,
43,147,154 γ
c,
43 and an IL-15-specific protein, IL-15Rα. IL-15Rα shares a number of structural similarities with IL-2Rα, including that it is a sushi domaincontaining protein (IL-2Rα has two sushi domains, whereas IL-15Rα has one),
155 and the
IL2RA and
IL15RA genes are closely positioned on human chromosome 10p14.
156 A distinctive feature of IL-15 signaling is that it signals substantially by a process called
transpresentation, wherein IL-15Rα on the surface of dendritic cells or monocytes will transpresent IL-15 to responding cells such as CD8+ T cells or NK cells that express IL-2Rβ + γ
c.
148,157 IL-15Rα has a longer cytoplasmic domain than IL-2Rα and appears to be critical for IL-15Rα function, although potentially not for transpresentation.
158 Interestingly, transpresentation can be utilized by IL-2 as well.
159 The very high affinity of IL-15Rα for IL-15 is explained by a large number of ionic interactions mediated by the sushi domain.
160 Note that these responding cells can also express IL-15Rα. In contrast to IL-2, which is a growth factor as well as a mediator of antigen-induced (or activationinduced) cell death and promoter of T
reg differentiation, the
role of IL-15 appears to be more focused on growth of CD8+ T cells,
161 maintaining long-lasting, high-avidity T-cell responses to foreign pathogens (ie, CD8+ T-cell memory).
148,162 This ability has suggested it could have potential as a vaccine adjuvant.
163 Overall, the fact that IL-15 promotes the proliferation and differentiation of B, T, and NK cells; the cytolytic activity of CD8 T cells; and the maturation of dendritic cells, yet, unlike IL-2, fails to stimulate immunosuppressive T
reg cells indicates that it has an array of properties that make it potentially promising as an anticancer therapeutic agent; as a result, a number of ongoing clinical trials are now in progress.
164IL-21 is the most recently identified member of the IL-2 family of cytokines. IL-21 can bind to specific receptors and exert actions on T, B, NK cells, DCs, and macrophages.
165 It augments T-cell proliferation as a comitogen,
166 can cooperate with IL-7 or IL-15 to drive the expansion of freshly isolated mouse CD8+ T cells, and it can augment the antitumor activity of CD8+ T cells.
167 IL-21 promotes the differentiation of Th17 cells
168,169 as well as the generation of T follicular helper cells.
170 In the case of Th17 differentiation, IL-21 is part of an IL-6 to IL-21 to IL-23 signaling cascade
169,171,172 to drive the differentiation of these cells in an RORγ-dependent manner.
173 Polarization of Th17 cells is dependent on TCR stimulation, TGF-β, and IL-6 but is independent of IL-23, which instead may be required for maintaining/expanding these cells.
174,175,176,177The actions of IL-21 on B cells are particularly complex. It augments B-cell proliferation when combined with anti-CD40 or lipopolysaccharide (LPS) but inhibits proliferation in response to anti-IgM + IL-4
166; this inhibition is reversed if anti-CD40 is additionally provided. It induces apoptosis of incompletely activated B cells, perhaps serving a role analogous to that of IL-2 in activation-induced cell death of T cells to eliminate incompletely activated cells.
165 In contrast, IL-21 drives terminal differentiation to plasma cells of more fully activated cells. Strikingly, IL-21 can drive plasma cell differentiation of both peripheral memory B cells and cord blood B cells, at least in part explained by its ability to induce expression of BLIMP1.
178,179 Strikingly, IL-21 regulates not only BLIMP-1 but also a broad range of genes via a functional cooperation between STAT3 and IRF4.
180 In IL-21R knockout mice, following immunization, IgG1 is diminished related to a role for IL-21 in class switching to IgG1 and IgG3, whereas IgE is elevated, related to the ability of IL-21 to inhibit Cε transcription
181 and/or to its ability to augment the apoptosis of IgE-producing B cells. Analysis of IL-21R/IL-4 double knockout mice has revealed that IL-21 cooperates with IL-4 to globally regulate Ig production in that these mice exhibit a panhypogammaglobulinemia, mimicking the T-cell phenotype in humans with X-linked SCID.
182 IL-21 can also cooperate with IL-15 and Flt-3 ligand to increase development of NK cells
166 and augment antitumor activity
183; however, it was also reported to oppose the actions of IL-15
184 and can also direct NK cell apoptosis. Interestingly, IL-21 exerts potent antitumor effects, including against large established solid tumors.
11,165 In the pMEL-1 CD8 TCR transgenic system, the effector cells recognize a melanoma antigen. When cells are adoptively transferred into tumor-bearing mice and animals are vaccinated with the cognate antigen, the addition of IL-21 or IL-21 plus IL-15 has potent antitumor effects.
167 Moreover, treatment of cells with IL-21 in vitro prior to adoptive transfer results in markedly enhanced antitumor effects in vivo, conferring a distinctive differentiation program from that conferred by IL-2.
185 IL-21 is now in phase II clinical trials for cancer. In addition to its anticancer activity, IL-21 can also promote autoimmunity. Elevated IL-21 levels have been reported in the BXSB-
Yaa mouse model of systemic lupus erythematosus,
178 and elevated IL-21 levels have been found in a subset of humans with systemic lupus erythematosus (P. Lipsky, personal communication). Elevated IL-21 has also been associated with other autoimmune processes, including in the non-obese diabetic (NOD) mouse.
181 More importantly, in mouse models of type 1 diabetes, lupus, and experimental allergic uveitis, no autoimmune disease develops on an IL-21R knockout background.
186,187,188 IL-21 unexpectedly can also be immunosuppressive via its induction of IL-10.
189,190 Consistent with this, an IL-21/IL-10/STAT3 pathway is required for normal development of memory CD8+ T cells after lymphocytic choriomeningitis virus (LCMV) infection
191; similarly, STAT3, presumably by an overlapping mechanism is important for human T-cell memory development, as evidenced by studies in patients with autosomal dominant hyper-IgE syndrome, which is caused by dominant negative mutations in STAT3.
192 IL-21 has complex roles in viral responses. For LCMV, IL-21 is required to control chronic infection,
193 whereas IL-21 mediates the pathogenic response after infection with pneumonia virus of mice.
194 Interestingly, for hepatitis B virus, IL-21 appears to be critical in determining the age-dependent effectiveness of immune responses, wherein decreased IL-21 production in younger individuals hinders the generation of critical lymphoid responses to the virus, whereas more robust IL-21 production in adults is associated with viral clearance that occurs in 95% of patients.
195The receptor for IL-21 consists of IL-21R plus γ
c.
30,44 IL-21R is most related to IL-2Rβ, and like IL-2Rβ, its expression is induced following cellular stimulation with anti-CD3 or phytohemagglutinin, and in addition, its expression is augmented in T cells following transformation with HTLV-I.
196 Both human and mouse IL-21 can act on cells of the other species. IL-21 is on human chromosome 4q26 to 4q27, while its receptor is on chromosome 16p11, immediately downstream of the
IL4R gene.
Thus, IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 collectively exhibit partially overlapping roles related to T cells, NK cells, B cells, and mast cells, and together would be expected to play vital roles for normal development and/or function of these cellular lineages. As discussed in the following, IL-2, IL-7, IL-9, and IL-15 utilize primarily STAT5A and STAT5B; IL-4 activates primarily STAT6; and IL-21 activates STAT1, STAT3, STAT5A, and STAT5B, with STAT3 being the dominant STAT for this cytokine.
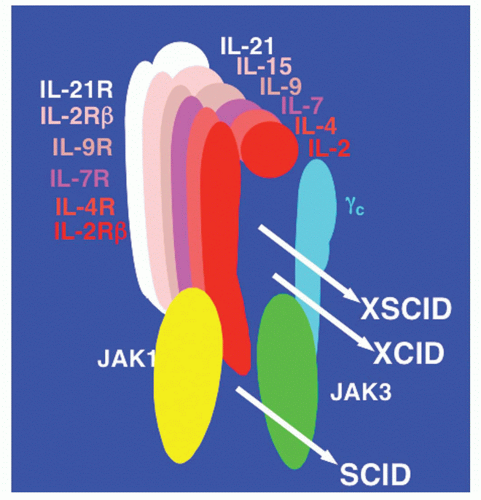
11 The fact that these six cytokines share γ
c is of particular interest, especially as the gene encoding γ
c is mutated in patients with the most common form of severe combined immunodeficiency in humans.
X-Linked Severe Combined Immunodeficiency Disease Results from Mutations in the Gene Encoding γc
The γ chain was originally identified as a third component of the IL-2 receptor
36 after it became clear that IL-2 receptor α and β chains alone were not sufficient to transduce an IL-2 signal. The hypothesis that the γ chain was a shared component of receptors for cytokines in addition to IL-2 was motivated from a comparison of the clinical phenotypes in humans that result from defective expression of IL-2 versus. the γ chain. In 1993, it was discovered that mutations in the gene encoding the γ chain resulted in X-linked SCID (the disease is also designated as SCIDX1).
30,197 X-linked SCID is characterized by profoundly diminished numbers of T cells and NK cells
30,45,197,198,199,200,201 (Table 25.4). Although the B cells are normal in number, they are nonfunctional, apparently due to a lack of T-cell help as well as an intrinsic B-cell defect.
45,200,202 In contrast to the profoundly decreased number of T cells in patients with X-linked SCID, IL-2-deficient patients
203,204 and mice
205 have normal numbers of T cells (the phenotypes of mice deficient in type I and type II cytokines and their receptor, JAK kinases, and STAT proteins are summarized in
Table 25.15). This observation indicated that defective IL-2 signaling was unlikely to be responsible for X-linked SCID, making the finding that the gene encoding the γ chain was mutated in X-linked SCID all the more unexpected. Thus, the conundrum was why a defect in a component of a receptor would cause a more severe than a defect in the corresponding cytokine. This led to the hypothesis that the γ chain was critical for other cytokine receptors as well.
197 In this model, defective IL-2 signaling either did not contribute to the defects in X-linked SCID or these defects were explained by the simultaneous inactivation of multiple signaling pathways.
45,197 Indeed, it was found that the γ chain was also an essential component of both the IL-4 and IL-7 receptors on T cells,
37,38,39,40 leading to it being renamed as the common cytokine receptor γ chain, γ
c.
37,38 IL-9, IL-15, and IL-21 were subsequently also shown to share γ
c30 (Fig. 25.5).
The sharing of γ
c by six different cytokine receptors revealed that X-linked SCID is a disease of defective cytokine signaling. The major deficiencies in X-linked SCID can be attributed to defects related to different cytokines. Based on the dramatically diminished T-cell development not only in IL-7-deficient
104 or IL-7Rα-deficient
103 mice but also in
IL-7R-deficient humans with T-B+NK+ SCID,
206,207 yet normal T-cell development in mice deficient in IL-2,
205 IL-4,
208,209 both IL-2 and IL-4,
210 IL-9,
133 IL-15,
151 IL-15Rα,
150 IL-21R,
182,184 and most if not all of the defect in T-cell development in patients with X-linked SCID can be attributed to defective IL-7 signaling.
30 In addition to profoundly diminished numbers of T cells, humans with X-linked SCID lack NK cells. As discussed previously, NK-cell development is defective in IL-15- and IL-15Rα-deficient mice, indicating that it is defective IL-15 signaling that is responsible for the defective NK-cell development in X-linked SCID.
30In contrast to the greatly diminished number of T cells and absent NK cells in patients with X-linked SCID, B-cell numbers are normal. This is in contrast to the greatly diminished numbers of B cells in γ
c-deficient mice
211,212 as well as mice deficient in either IL-7 or IL-7Rα, indicating that IL-7 is not required for pre-B-cell development in humans and underscoring a major difference for IL-7 in human versus mouse biology. Indeed, patients with
IL-7R-deficient SCID have a T-B+NK+ form of SCID.
206,207 Although B cells develop in patients with X-linked SCID, they are nonfunctional. This is due in part to a lack of T-cell help (given the near absence of T cells in X-linked SCID), but a variety of data indicate an intrinsic B-cell defect as well.
30 As discussed
previously, analysis of IL-4/IL-21R double knockout mice indicate that defective signaling by IL-4 and IL-21 appear to explain the intrinsic B-cell defect in X-linked SCID.
182
Rationale for the Sharing of γc
Why should there have been evolutionary pressure to maintain the sharing of γ
c, given the obvious increased risk associated with sharing a receptor component when it is mutated? There are at least two different types of models.
45,213 First, the sharing of γ
c could be a basis for shared actions. For example, given that IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 can each act as T-cell growth factors, at least in vitro, it is possible that γ
c might couple to signal transducing molecule(s) that promote T-cell growth. Second, the sharing of γ
c might represent a means by which one cytokine using γ
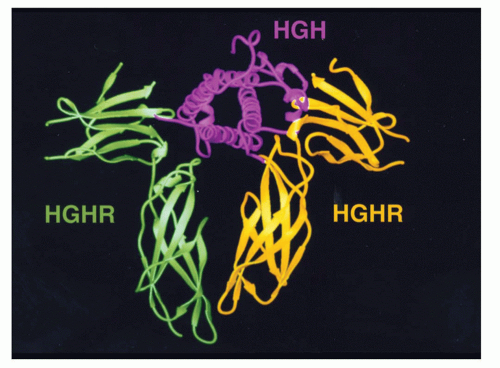
c can modulate the signals of the others. To understand this model, it is important to recognize that, in contrast to antigen receptor complexes, cytokine receptor components individually are targeted to the cell surface, and the formation/stability of receptor complexes is promoted or stabilized by ligand binding, as noted in the discussion of growth hormone, wherein the second receptor monomer recognizes the combined surface of growth hormone and the first growth hormone receptor monomer
19 or for the IL-2 receptor where γ
c “sees” a combined IL-2/IL-2Rβ surface.
23 This latter finding is consistent with γ
c originally having been coprecipitated with IL-2Rβ in the presence but not in the absence of IL-2,
214 and dimerization of IL-2Rβ and γ
c is known to be required for efficient signaling.
215,216 Thus, receptor heterodimerization at a minimum is stabilized by the cytokine and physiologically may be absolutely dependent on the presence of the cytokine. In the absence of stable preformed cytokine receptor complexes between γ
c and the other receptor chains, one can envision that γ
c might be differentially recruited to different receptors based on the relative amount of a cytokine or its binding efficiency. In a situation where γ
c is limiting, a cytokine might then not only induce its own action but could also simultaneously inhibit the action of another cytokine that was less efficient at recruiting γ
c to its cognate receptor complex.
An analysis of mice deficient in IL-2,
205 IL-2Rα,
217 IL-2Rβ,
218 and γ
c211,212 provides the interesting observation that although the mice lacking γ
c have defective signaling in six different cytokine pathways, mice deficient in IL-2, IL-2Rα, and IL-2Rβ appear to be less healthy than the γ
c-knockout mice with more markedly activated T cells and autoimmunity that is not evident in γ
c-deficient mice. This is at least in part explained by the fact that IL-2-deficient mice lack T
reg cells and thus develop autoimmune disease,
65 whereas γ
c-deficient mice lack T cells due to defective IL-7 signaling and thus lack effector T cells. These and other data indicate that γ
c plays a major role in regulating lymphoid homeostasis, as originally indicated.
219
Cytokines whose Receptors Share the Common β Chain, βc (Interleukin-3, Interleukin-5, and Granulocyte Macrophage-Colony Stimulating Factor)
The hematopoietic cytokines, IL-3, IL-5, and GM-CSF
(Table 25.5) are all synthesized by T cells and exert effects on cells of hematopoietic lineage.
220,221,222 These cytokines are vital for proliferation as well as differentiation of myeloid precursor cells. Of these three cytokines, IL-3 is the most pluripotent
221 and historically was also called multi-CSF, reflecting the large number of lineages on which it can act. It can act to promote proliferation, survival, and development of multipotent hematopoietic progenitor cells and of cells that have become dedicated to a range of different lineages, including granulocyte, macrophage, eosinophil, mast cell, basophil, megakaryocyte, and erythroid lineages. It induces a range of effects, including, for example, inducing the production off IL-4 by basophils.
223 IL-3 also can exert end-function effects, such as enhancing phagocytosis and cytotoxicity. GM-CSF is mainly restricted to the granulocyte and monocyte/macrophage lineages, but its actions are nevertheless still quite broad.
220 It is both a growth and survival factor. In addition, it can expand the number of antigen-presenting cells, such as DCs, and thereby may greatly expand the ability of the host to respond to antigen. IL-23 and RORγt can augment GM-CSF production in Th cells, whereas IL-12, IFNγ, and IL-27 negatively regulate its expression, and GM-CSF is now known to be required for the initiation of autoimmune neuroinflammation in experimental autoimmune encephalitis. Collectively, these results suggest that GM-CSF is important for encephalitogenic actions of both Th1 and Th17 cells.
224,225 GM-CSF mediates autoimmune effects at least in part by enhancing IL-6-dependent
Th17 cell development and survival.
226 Whereas IL-3 and GM-CSF can act on eosinophils, they act at much earlier stages than IL-5, presumably expanding the number of eosinophil-committed precursors cells. IL-5 stimulates the eosinophilic lineage and eosinophil release from the bone marrow and is essential for expanding eosinophils after helminth infections,
227,228 and can mediate the killing of
S. mansoni. IL-5 can also induce Ig production in B cells activated by contact with activated Th cells in mouse systems, and IL-5 and IL-5Rα knockout mice have diminished CD5+ B1 cells and decreased thymocytes until approximately 6 weeks of age.
227,228 Interestingly, the production of IL-5 by memory Th2 cells is driven by GATA3 but downregulated by expression of eomesodermin, which interacts with GATA3. Most memory Th2 cells produce the transcription factor Eomes, but the CD62
loCXCR3
lo population has decreased Eomes and is the population of cells that produces IL-5.
229On cells that express receptors for more than one of these cytokines, such as eosinophilic progenitors that express receptors for IL-3, IL-5, and GM-CSF, or on mouse pre-B cells, which express receptors for IL-3 and IL-5, the signals induced are indistinguishable.
221,222 Thus, the differential lineage specificities of these cytokines are determined by the cellular distribution of their receptors rather than by fundamental differences in the signals that are induced by each cytokine. These observations are explained by studies demonstrating that each of these three cytokines has its own unique 60 to 80 kDa α chain (ie, IL-3Rα, IL-5Rα, and GM-CSFRα),
230,231,232,233,234,235,236 but that they share a common 120 to 130 kDa β chain, β
c.
222,237,238,239 The α chains are the principal binding proteins for the cytokines, whereas the shared β
c subunit augments binding affinity but does not exhibit binding activity in the absence of the proper α chain. The α chains have relatively short cytoplasmic domains (approximately 55 amino acids long for IL-3Rα, IL-5Rα, and GM-CSFRα) and are not believed to play major roles in signaling function, whereas β
c, with its cytoplasmic domain of 432 amino acids, is the primary determinant of the signal. As a result, there is a relative compartmentalization of binding and signaling function for these cytokines, although the cytoplasmic domains of the GM-CSFRα and IL-5Rα chains (and by analogy, perhaps the IL-3Rα chain), as well as β
c, appear to be capable of at least modulating the growth signals in transfected cells.
222,240,241,242 In any case, the sharing of β
c helps to explain why the signals induced by IL-3, IL-5, and GM-CSF are similar on cells that can respond to more than one of these cytokines. The situation for the β
c family of cytokines is therefore quite different from the receptors for γ
c family cytokines, wherein the chains with the largest cytoplasmic domains (IL-2Rβ, IL-4Rα, IL-7Rα, IL-9Rα, and IL-21R) not only contribute most to signaling specificity but also are the proteins principally involved in ligand binding (note that for IL-2 and IL-15, IL-2Rα and IL-15Rα, respectively, serve important roles as well). The shared chain, γ
c, serves a vital accessory function (hence the development of X-linked SCID when the
IL2RG gene is mutated), but it contributes less to cytokine binding and does not provide an obvious basis for signaling specificity (see subsequent discussion).
An interesting feature of the β
c family of hematopoietic cytokines is that there appears to be considerable redundancy of function so that knockout mice that lack the ability to respond to all three cytokines (GM-CSF, IL-3, and IL-5) due to deletion of β
c as well as the mouse IL-3-specific β
c-like protein (discussed in the following text) nevertheless exhibit relatively normal hematopoiesis. These observations do not minimize the potency of these particular cytokines but instead underscore a substantial redundancy for a particularly important set of functions.
221,222,243 It is also noteworthy that β
c-deficient mice exhibit defective host responses to infectious challenge, suggesting that these hematopoietic cytokines play a vital role in promoting immune function.
Cytokines whose Receptors Share gp130 (Interleukin-6, Interleukin-11, Oncostatin M, Ciliary Neurotropic Factor, Leukemia Inhibitory Factor, Cardiotrophin-1, Novel Neurotrophin-1/B Cell-Stimulating Factor-3/Cardiotrophin-Like Factor, and Interleukin-27)
There are now eight cytokines that are known to utilize gp130 as a signal transducing molecule.
244,245,246,247,248,249,250,251,252,253,254,255 Some of the properties of these cytokines are summarized in
Table 25.6. This family is often referred to as the IL-6 family of cytokines and includes IL-6, IL-11, OSM, LIF, CNTF, CT-1, NNT/BSF-3/CLC, and IL-27. This group of cytokines comprises molecules with a diverse range of actions, ranging beyond the hematopoietic and
immune systems to the central nervous and cardiovascular systems, making them even more “multifunctional” than the γ
c and β
c families of cytokines, whose actions are more restricted to the lymphoid and hematopoietic systems.
IL-6 was originally identified and then cloned as a B-cell differentiation factor that stimulated terminal differentiation/maturation of B cells into antibody-producing plasma cells.
256,257,258 However, IL-6 also can exert effects for T-cell growth and differentiation (and thus is a thymocyte “comitogen”), induce myeloid differentiation into macrophages, induce acute-phase protein synthesis of hepatocytes, and exert actions on keratinocytes, mesangial cells, hematopoietic stem cells, the development of osteoclasts, and neural differentiation of PC12 cells.
250 IL-6 is also a mediator of amplified lymphocyte trafficking during febrile inflammatory responses, which is dependent on IL-6-mediated activation of L-selectin.
259 IL-6 binds to an 80 kDa IL-6 binding protein, denoted IL-6Rα, which has a comparatively short 82 amino acid long cytoplasmic domain.
257,260 This IL-6-IL-6Rα complex then interacts with and recruits the 130 kDa signal transducing molecule, gp130, which together with IL-6Rα can form a functional IL-6 receptor.
250 From a structural perspective, gp130 contains a total of six fibronectin type III modules, with the four conserved cysteine residues and the WSXWS motif being located in the second and third of these modules, starting from the N-terminus. As such, these regions are topologically positioned at greater distance external to the cell membrane than is the case for the other type I cytokine receptors discussed previously.
The IL-6 system illustrates a novel twist related to the properties of their principal binding proteins: The cytoplasmic domain of IL-6Rα is superfluous for signaling; a soluble form of the IL-6Rα extracellular domain is in fact sufficient for ligand binding and coordination with gp130, a process termed
IL-6 transsignaling.
261 Thus, in the presence of soluble IL-6Rα and IL-6, many cell types that express gp130 but not IL-6Rα are capable of signaling in response to IL-6. It was observed that IL-6 signaling requires the dimerization of gp130 and that the overall complex containing two molecules each of IL-6, IL-6Rα, and gp130 (a dimer of a trimer or a hexamer),
32,262,263,264,265 providing a possible paradigm for the stoichiometry of subunits for other members of the IL-6 family of cytokines.
24IL-11 was identified as a factor produced by a stromal cell line in response to stimulation with IL-1.
266,267 It has a number of effects on hematopoiesis, particularly in combination with IL-3 and SCF. Because IL-11 exhibited “IL-6-like activities,” a complementary deoxyribonucleic acid (DNA) was isolated based on the presence of IL-6-like activity in the presence of antibodies to IL-6.
268 Other actions of IL-11 include the ability to stimulate the proliferation of lymphoid and hematopoietic progenitor cells, stimulate megakaryocytic progenitors and megakaryocyte maturation, and stimulate erythroid progenitors (an action not shared by IL-6).
266,267 Overall, it acts on T cells, B cells, hematopoietic cells, epithelium, endothelial cells, and osteoclasts.
269 Like IL-6, IL-11 induces acute-phase proteins and augments antigen-specific B-cell responses, but it does not stimulate human myeloma cells.
266,267,268,270 Subsequently, adipogenesis inhibitory factor was cloned and found to be identical to IL-11,
271 revealing another action of IL-11. IL-11 is also produced by lung eosinophils and various structural cells in the lung and is expressed in patients with modest-to-severe asthma.
272 IL-11 signals via a receptor complex containing both IL-11Rα
273 and gp130.
244 Interestingly, IL-11Rα messenger RNA can be alternatively spliced to yield a form lacking the cytoplasmic domain, and like IL-6Rα, a soluble form of IL-11Rα can coordinate with IL-11 to transsignal in cells expressing gp130.
274 Studies on the stoichiometry of the IL-11 receptor complex failed to reveal dimerization of gp130 to itself or LIFRβ. Thus, assuming that it forms a hexameric receptor complex, only five of the members are known: two molecules of IL-11 and IL-11Rα, respectively, and one of gp130, suggesting that another component may still be found.
275 IL-11 and STAT3 are expressed in gastrointestinal cancers associated with inflammation, suggesting a possible link to cancer.
269LIF is another multifunctional cytokine originally cloned based on the activity associated with its name.
276 LIF can suppress the differentiation of pluripotent embryonic stem cells, inhibit adipogenesis (like IL-11), and induce monocyte differentiation of the M1 murine leukemia cell line, thus mimicking a number of the actions of IL-6.
277 In addition, it exerts a number of actions in the central nervous system, and LIF was shown to be identical to cholinergic neural differentiation factor.
278 and can induce acetylcholine synthesis while simultaneously suppressing catecholamine production, thereby inducing cholinergic function while suppressing noradrenergic function.
278 LIF has been shown to be essential for embryo implantation.
279 LIF binds to a receptor (LIFRβ) that is structurally related to gp130,
280 but the functional LIF receptor (LIFR) receptor requires the heterodimerization of LIFRβ and gp130 as well.
246 Interestingly, whereas IL-6 promotes Th17 differentiation, this is inhibited by LIF, apparently based on LIF’s ability to activate ERKs and to augment the expression of SOCS-3, which can inhibit STAT3 activation.
281 Interestingly, LIF can also promote the differentiation of T
reg cells and may exhibit therapeutic potential in multiple sclerosis.
282 LIF maintains mouse embryonic stem cells, but LIF is not capable of maintaining human embryonic stem cells or mouse epiblast stem cells in the pluripotent state.
283CNTF was discovered based on its ability to promote neuronal survival.
284,285 CNTF signals through a receptor comprising LIFRβ and gp130 but requires a specific binding protein,
286,287 now denoted CNTF receptor (CNTFR)α. Interestingly, CNTFRα lacks transmembrane and cytoplasmic domains and instead is a GPI-linked receptor molecule. CNTFRα appears to provide a receptor-cytokine surface with which gp130 and LIFRβ can interact. Thus, CNTF is like IL-6 in that each requires initial binding to a receptor component (CNTFRα or IL-6Rα) that does not require its own cytoplasmic domain for signaling. Whereas IL-6 signaling involves homodimerization of gp130, CNTF signaling involves the heterodimerization of LIFRβ and gp130. In fact, the functional CNTFR appears to be a hexameric structure containing two molecules of CNTF, two of CNTFRα, and one each of gp130 and LIFRβ.
288 The receptor is expressed largely within the nervous system and in skeletal muscle, accounting for largely restricted actions of CNTF.
286OSM is a growth regulator that was originally identified based on its ability to inhibit the growth of A375 human melanoma cells.
289,290 OSM is a potent growth factor for Kaposi sarcoma in patients with acquired immunodeficiency syndrome.
291,292 Not only OSM can bind directly to gp130 and signals through a receptor combination of gp130 and LIFRβ
246 but also has an alternative receptor comprising a specific OSM receptor subunit (OSMRβ) and gp130.
293 These are now known as the type I and type II OSM receptors (OSMRs), respectively. OSM can enhance the development of both endothelial cells and hematopoietic cells, possibly by increasing hamangioblasts, a common precursor for endothelial and hematopoietic cells.
251CT-1 was initially isolated based on its actions on cardiac muscle cells.
294 However, it is now clear that it is a multifunctional cytokine with hematopoietic, neuronal, and developmental effects, in addition to its effects on cardiac development and hypertrophy.
295,296 Like OSM and LIF, CT-1 can also signal through a heterodimer of LIFRβ and gp130.
249 Interestingly, the CT-1 receptor on motor neurons may involve a third receptor component, possibly GPI linked.
297,298NNT-1/BSF-3, like CNTF, can also support the survival of chicken embryonic sympathetic and motor neurons.
253 Interestingly, in mice, NNT-1/BSF-3 can augment the effects of IL-1 and IL-6 and is a B-cell-stimulating factor (hence the term BSF-3). The NNT-1 receptor contains LIFRβ and gp130.
253 NNT-1 is also known as CLC, and this forms a complex with a soluble receptor protein known as cytokine-like factor-1. Together, this complex is a second ligand for CNTFR.
299 It appears that CLC can also interact directly with soluble CNTFR to form a related cytokine.
254IL-27 is an IL-6-related cytokine
300 that represents a dimer of the p28 protein (also known as IL-30) and EB13 (Epstein-Barr virus-induced gene 3) (also known as IL-27B), which can induce proliferation of naive CD4+ T cells. p28 can exist by itself independently of EB13 and is capable of antagonizing gp130-mediated signaling by IL-6, and indeed, overexpression of p28 in transgenic mice prevents normal development of germinal centers and antibody production.
301 Together with IL-12, IL-23, CLC/cytokine-like factor-1, and CLC/soluble CNTFR, this is one of five cytokines that represent dimers including a type I cytokine and a soluble receptor-like protein. IL-27 signals via gp130 and the WSX-1/TCCR receptor,
300 which is discussed in the section on diseases of cytokine receptors and related molecules. IL-27 has proinflammatory and anti-inflammatory effects. Although it was suggested to promote Th1 responses, it is clear that it can also antagonize such responses, and IL-27 can promote effector responses of both CD4+ and CD8+ T cells, of NK cells, and it additionally stimulates mast cells. The anti-inflammatory effects of IL-27 are indicated by the absence of WSX-1 resulting in increased mast cell and macrophage responses.
302,303Thus, eight cytokines (IL-6, IL-11, LIF, CNTF, OSM, CT-1, NNT-1/BSF-3, and IL-27) all have receptors that are dependent on gp130.
304 These can be divided into two sets of cytokines: those known to not require LIFRβ (IL-6, IL-11, and IL-27) and those that use both gp130 and LIFRβ (LIF, CT-1, OSM, CNTF, and NNT-1/BSF-3)
(Table 25.7), with OSM having two forms of receptors, each of which contains gp130 but only one of which contains LIFRβ. When cytokines share essentially the same receptor, one can hypothesize that two cytokines might exert identical actions on cells that can respond to both cytokines. It is clear that the presence of IL-6Rα, IL-11Rα, and CNTFRα (either on the cell surface or as a soluble receptor form, discussed subsequently) determines whether a cell can respond to IL-6, IL-11, and CNTF. This raises the interesting question as to whether functional homologues of these proteins will also exist for LIF, OSM, CT-1, NNT-1/BSF-3, and IL-27.