Despite its length and surface area, the small bowel accounts for less than 5% of all gastrointestinal cancers, and has a malignancy rate 50-fold lower than that of the colon.1–3 When tumors do arise in the small bowel, however, they tend to evade detection for some time and therefore commonly present at an advanced stage. This chapter will review the most common types of small bowel neoplasms and their management, including adenocarcinoma, gastrointestinal stromal tumor, and lymphoma, while excluding tumors of the duodenum and neuroendocrine tumors, which are covered elsewhere in this volume.
For many years adenocarcinoma was the predominant primary neoplasm of the small bowel, but has recently been supplanted by small bowel neuroendocrine tumors.1,3 Excluding the duodenum, adenocarcinoma represents approximately 24% of primary small bowel cancers,1 and has an annual incidence of 2000 to 3000 cases per year in the United States.3 Small bowel adenocarcinoma (SBA) tends to afflict older patients. Surveillance, Epidemiology, and End Results (SEER) program data indicate that more than 85% of SBA patients are older than 50 years, with a median age at diagnosis 67 years.2,3 Due to its rarity, nonspecific symptoms, and lack of screening tools, SBA presents at a later stage than colon cancer. A recent comparison of large bowel with SBA from SEER data found that 32% of SBA presents with stage IV disease, whereas only 20% of colonic adenocarcinomas have metastases at diagnosis.3
Processes causing increased inflammation predispose to SBA. Crohn’s disease affecting the small bowel is a strong risk factor for development of SBA, and risk increases with greater duration of symptoms.4 Celiac disease also confers higher risk of SBA.5 Smoking, alcohol use, diets high in animal fats, cystic fibrosis, male gender, and the presence of additional GI cancers have also been correlated with increased SBA risk.2,6,7 Patients with familial polyposis syndromes, such as familial adenomatous polyposis (FAP), hereditary nonpolyposis colorectal cancer (HNPCC), and Peutz–Jeghers syndromes have a high risk of developing small bowel polyps and adenocarcinomas.2
Similar genetic alterations occur in colorectal adenocarcinoma and SBA, with some important differences. Loss of tumor suppressor genes and activating mutations in oncogenes occur in both cancer types, including p53, SMAD4, KRAS, and β-CATENIN.2,7 KRAS mutations were found in 43%, and p53 overexpression in 42% of SBA tumors in one series, similar to rates observed in colorectal adenocarcinomas.8 Interestingly, nonsense mutations in the APC tumor suppressor gene, which occur in 60% to 80% of colorectal adenocarcinomas, are not found in SBA.7 Instead, missense APC mutations are common, occurring in 42% of sporadic SBAs and 7% of celiac-related SBAs.7 Patients with APC mutations have significantly worse survival than those without mutations.7 Accumulation of β-catenin in the nucleus, usually resulting from inactivation of APC, is observed in 80% of colorectal adenocarcinomas, but occurs in only 20% of SBAs, a rate similar to that found in gastric cancer.8 Deficiency in mismatch repair occurs in approximately 5% to 30% of SBAs, with higher rates in suspected Lynch-syndrome families, and may be associated with earlier stage and better prognosis than mismatch repair-intact tumors.8
Small bowel adenocarcinomas most commonly occur as well or moderately differentiated carcinoma, but a significant proportion of tumors (33% to 47%) are classified as poorly differentiated or signet-ring cell carcinomas, which are associated with worse prognosis.3,6,9 Lesions arise from the small bowel mucosa, and can present as exophytic masses causing obstruction, bleeding into the lumen, or with invasion or even perforation of the small bowel wall (Fig. 104-1).10
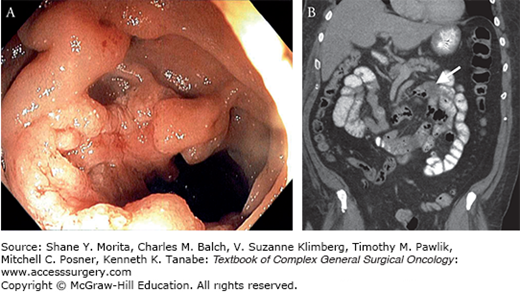
FIGURE 104-1
Small bowel adenocarcinoma. This patient presented with abdominal pain and GI bleeding. Push enteroscopy (A) revealed a fungating mass in the proximal jejunum just beyond the ligament of Treitz, which was also visible on CT scan (B) (solid lesion marked with arrow). The patient underwent successful resection of this T3N0 lesion.
The most common presenting symptom of SBA is abdominal pain. Combining data from two large retrospective studies, out of 708 SBA patients, the most common presenting symptoms were abdominal pain in 347 (43%), nausea, vomiting, or obstructive symptoms in 163 (23%), and anemia or GI bleeding in 157 (22%).6,9 Historically, diagnosis of SBA most often occurred at laparotomy undertaken for symptoms, but diagnosis by CT, usually obtained to evaluate abdominal pain, has become more common in the modern era.6,9 Upper and lower endoscopies are often performed due to bleeding, and should be used to rule out bleeding sources other than the small bowel. However, when bleeding does originate in the small bowel, conventional endoscopy cannot reach most tumors.2 Although capsule endoscopy and double-balloon enteroscopy have shown some success in locating SBAs, the diagnostic evaluation of choice for a suspected SBA has become CT enteroclysis.11 In this modality, rapid infusion of 2.5 L of water by nasojejunal tube accompanied by contrast-enhanced CT has shown high accuracy (sensitivity 84.7%, specificity 96.9%, positive predictive value (PPV) 90.9%, and negative predictive value (NPV) 94.5% in a study of 219 patients) in locating suspected masses in the small bowel.11 CT enteroclysis also provides staging information by simultaneously evaluating for liver metastases. Still, once a mass has been identified, tissue diagnosis is often difficult prior to surgery due to its location.
With suspected or tissue-confirmed SBA, a complete staging workup includes CT of the abdomen and pelvis and either CT or x-ray of the chest to assess for liver and lung metastases.10 Staging of SBA follows the AJCC TNM staging system for small bowel cancer. Depth of invasion determines T-status. T1 tumors are confined to the submucosa, T2 tumors enter the muscularis propria, T3 tumors grow into the subserosa, while T4 tumors penetrate the visceral peritoneum or directly invade other organs. Stage I tumors are T1-2N0M0, stage II tumors are T3-4N0M0, stage III tumors have nodal metastases, and stage IV tumors have distant metastases. Stages II and III are further divided into A and B categories based on T3 versus T4 for stage II and N1 versus N2 (more than three positive regional lymph nodes) for stage III.12
While survival rates of upper and lower GI cancers have shown improvement, possibly due to improved detection of early-stage cancers, SBA survival rates have remained largely stable over time.3 In general, prognosis is poor due to the advanced stage at which many tumors are discovered. An analysis of SEER results from 1973 to 2005 found 5-year survival rates for stage I–IV disease of 60%, 40%, 27%, and 3.2%, respectively, similar to earlier findings using the National Cancer Database.13,14 Jejunal tumors may carry a slightly better prognosis than those of the ileum.13 Median survival for stage III and IV (which include the majority of SBA patients) was approximately 2 years and 1 year, respectively, in a large retrospective study.9 Surgical intervention is associated with improved survival versus no surgery, and complete resection (R0) confers improved survival compared to incomplete resection.1,3,9
Management of localized SBA is surgical. Institutional and population-based studies identify surgical resection as a strong determinant of improved survival in SBA.3,9 Retrospective studies have also shown significant understaging of SBA associated with resection of fewer than 8 to 10 lymph nodes and better outcomes in patients with more nodes resected.3,13 Therefore, complete R0 tumor resection with adequate lymphadenectomy is essential in SBA treatment. The affected small bowel should be segmentally resected along with at least 10 cm of healthy small bowel on either side of the tumor, with the associated mesentery up to the segmental branches coming off of the superior mesenteric artery and vein. For tumors arising in the distal ileum, right hemicolectomy is often required.9 After resection, primary anastomosis should be performed.
For patients presenting with advanced or metastatic disease, optimal management is unclear due to rarity of SBA and corresponding lack of clinical trials. Neoadjuvant chemotherapy may be considered for attempted downstaging of locally advanced disease, but this is unsupported by randomized data.10 Resection of isolated liver metastases by analogy to colorectal cancer may also be appropriate in highly selected patients, but again, no rigorous data exist.
Recurrent SBA most often appears in the liver, followed by abdominal carcinomatosis.6 Although no randomized trials of adjuvant chemotherapy exist, treatment is often by analogy to other GI cancers, where platinum-based agents and fluoropyrimidines show adequate response rates.9 Two phase II trials tested such regimens for primary treatment of SBA patients with locally advanced or metastatic disease. The first phase II trial treated 31 patients, including 11 with nonduodenal SBA with capecitabine and oxaliplatin, and demonstrated an objective response rate of 50%, with a median survival of 20.3 months among all patients and 15.3 months in patients with stage IV disease.15 Another trial tested FOLFOX in 33 patients, including 7 nonduodenal SBA patients, and found a similar response rate of 49% and overall survival of 15.2 months. Nonduodenal SBA patients had median survival of 13.0 months.16 Thus, although randomized data are lacking, case reports and nonrandomized trials support the use of these regimens as first-line treatment in unresectable SBA, as well as for adjuvant treatment of patients with incomplete resection or disease with a high risk of recurrence.
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the GI tract.17–43 Although most GISTs (approximately 70%) arise from the stomach, the second-most frequent site of origin (approximately 20% to 30%) is the small bowel.25 Small bowel GIST has an incidence of around 1200 cases per year in the United States,44 making GIST the third most common primary solid cancer of the small bowel (10% to 14% of all small bowel malignancies), after neuroendocrine cancers (45%) and adenocarcinoma (24%).1,14,28
Until the late 1990s, most GISTs were classified as a hodgepodge of other tumor types, including leiomyosarcomas, peripheral nerve sheath tumors, neurofibromas, and others.45 In 1998, expression of the tyrosine kinase receptor c-KIT, also known as CD117, in conjunction with CD34 was recognized as a hallmark feature of GISTs.46 GISTs shared expression of these markers with the interstitial cells of Cajal (ICCs), implicating ICCs as the previously unknown tissue of origin for GIST.46 ICCs are located in the myenteric plexus and muscularis propria of the bowel, where they serve as pacemaker cells, regulating peristalsis and autonomic activity.17,45 It was further recognized that GISTs frequently carried somatically occurring KIT mutations, which cause constitutive activation of the receptor, leading to unrestricted growth in cell culture and proliferation of tumors in vivo.46 Analyses of large series confirmed that most (>85%) GISTs carry activating mutations in KIT, with a minority (approximately 5%) having mutations in the closely related platelet-derived growth factor receptor alpha gene (PDGFRA).17,44,45 In small bowel GISTs, a series investigating mutations in 145 tumors found mutations in KIT only, with no mutations of PDGFRA, while 25% of small bowel GISTs were wild-type for both genes.30
These discoveries revolutionized treatment of GISTs because it was soon observed that the tyrosine kinase inhibitor imatinib, originally developed for use in chronic myelogenous leukemia against the BCR-ABL fusion protein of the Philadelphia chromosome, also inhibits mutant KIT and PDGFRA, and restricts growth of GIST cells.27,47–50 Initial reports of imatinib treatment in metastatic GIST were promising,25 and human trials of imatinib therapy for metastatic GIST began.26,51
The Intergroup S0033 phase III trial randomized 694 patients with inoperable metastatic GIST to either 400- or 800-mg daily imatinib therapy.26 While there was no significant difference between dose levels, patients enjoyed dramatically improved time to progression and overall survival than historical controls, with over 70% 2-year survival for both dose levels compared to 26% in previous GIST chemotherapy trials.26
Concurrently, to improve upon historically high rates of recurrence after operative resection of malignant GIST, which approached 50% at 5 years, the ACOSOG Z9000 and Z9001 trials evaluated the impact of adjuvant imatinib therapy.44,52 The Z9001 trial randomized 713 patients with completely resected, KIT-positive GISTs larger than 3 cm to 12 months of treatment with either 400 mg of imatinib daily or placebo.44 The original endpoint was overall survival, but due to the high response rates of patients in the placebo group crossing over to imatinib, the endpoint was changed to progression-free survival. The trial was halted in 2007 when interim analysis revealed efficacy in the treatment arm sufficiently high to make the placebo group no longer justifiable. With median follow-up of 19.7 months, recurrence in the imatinib group was 8%, versus 20% in the placebo group (p < 0.0001). Benefits were seen in tumors of all sizes, but the greatest benefit in recurrence-free survival was observed among tumors >10 cm, those at the highest risk of recurrence.44 A similar effect was seen in the Z9000 trial, a phase II nonrandomized trial of 12 months of adjuvant imatinib treatment in patients with localized, completely resected GISTs at high risk of recurrence.52 High-risk GISTs were defined as those greater than 10 cm in diameter, with intraperitoneal tumor rupture, or with peritoneal implants. With a median follow-up of 7.7 years, median recurrence-free survival was 4.0 years, and 73% of patients survived for 5 years.
With the evident success of imatinib treatment, the optimal duration of adjuvant therapy remained unknown. Longer follow-up of the Z9001 trial revealed that recurrences increased at 18 months—6 months after discontinuing treatment, which suggested that continuing treatment for longer periods could be beneficial.52 A Scandinavian/German trial attempted to answer this question by randomizing patients with resected, high-risk GIST to 1 versus 3 years of adjuvant imatinib treatment. With a median follow-up of 54 months, recurrence-free and overall survival were significantly better in the 3-year treatment group (5-year RFS 65.6% vs. 47.9% in the 1-year treatment group, p < 0.001; 5-year overall survival 92.0% in the 3-year treatment group vs. 81.7%, p = 0.02).18 This was the first trial to show improved overall survival after adjuvant treatment with a receptor tyrosine kinase inhibitor, and demonstrated that 3 years of imatinib is preferable in higher-risk GISTs.
Despite great progress in GIST treatment, small bowel GISTs consistently demonstrate worse outcomes than GISTs arising from the stomach or other sites.31 Small bowel GISTs tend to be larger at the time of diagnosis, but even when matched for size and mitoses, small bowel GISTs are more aggressive.45 Pathologic behavior is determined principally by size and mitotic rate, but location in sites other than the stomach is significantly associated with worse recurrence-free survival.19,30 Some of the difference in outcomes between small bowel GISTs and other locations may be due to tumor genotype. KIT mutations in GIST occur most often in exon 11, and with decreasing frequency in exons 9, 13, and 17.45 Exon 9 mutations, over 90% of which are a two residue alanine-tyrosine duplication at position 502-503,23 occur in around 5% to 13% of GISTs but are more common in small bowel GISTs.31,45 A review of 120 patients undergoing surgery for GIST at a single center prior to imatinib found that while small bowel GISTs accounted for 47% of the total study group, they included 77% of exon 9 mutations, and all patients with exon 9 mutations recurred.20 Whether exon 9 mutations confer a more aggressive phenotype or are simply more common in more aggressive small bowel tumors is unclear, as a large series of small bowel GISTs treated prior to imatinib found no difference in outcomes between patients with KIT exon 11 compared to exon 9 mutations.30 Nevertheless, GISTs carrying exon 9 mutations show worse response to imatinib. In trials of imatinib treatment for metastatic GIST, complete or partial responses occurred in 44.4% of patients with exon 9 mutations versus 71.7% of those with exon 11 mutations, and recurrence-free survival was worse with exon 9 mutations compared to tumors with the more common exon 11 mutations.23 Similarly, 3-year disease-free survival in patients treated with neoadjuvant and adjuvant imatinib was 52% for small bowel GISTs compared to 83% for gastric GISTs.21 GISTs with KIT exon 17 or PDGFRA D842V mutations show imatinib resistance, as do patients with wild-type KIT and PDGFRA.28,45 Mutations in KIT exon 17 occur twice as frequently in small bowel compared to gastric GISTs.53
Patients of all ages may be afflicted by small bowel GIST, but the vast majority (>85%) are older than 40 and the median age at diagnosis is 59.30 Bleeding is the most common presenting symptom, although intestinal obstruction, acute abdominal pain, and rarely hemodynamic instability due to intraperitoneal tumor rupture and hemorrhage occur.30,54 Anemia-related fatigue is a frequent presenting symptom, and large tumors may produce early satiety or a palpable abdominal mass.28,34 Small bowel GISTs may elude diagnosis for some time, and can be large in size at presentation. In a series of 906 small bowel GISTs, the median size at surgery was 7.0 cm (range 0.3 to 40 cm), and 64% were larger than 5 cm.30 Patients with neurofibromatosis type 1 are at elevated risk for small bowel GIST and may present with multifocal tumors.30,45 Familial GIST syndromes associated with germline KIT and PDGFRA mutations exist.22,30,35 Metastatic spread is by the hematogenous route, and metastases occur most commonly in the abdomen, followed by the liver.30
Evaluation of a suspected small bowel GIST is most efficiently accomplished by CT scan with IV contrast.28 An enhancing mass arising from the wall of the small bowel, often extending into the peritoneum, may be visualized, while the liver is also evaluated for metastases. Heterogeneity in the tumor may indicate hemorrhage or necrosis.34 GIST may be strongly suspected based on imaging features, and if resection is planned, avoiding preoperative biopsy minimizes the risk of tumor rupture.28,34 When a biopsy is required, FNA is preferred.28 Additional studies to consider include endoscopy/colonoscopy with endoscopic ultrasound, and magnetic resonance.28 FDG/PET offers little additional information beyond standard CT, and is infrequently indicated.28
Surgical therapy of small bowel GIST requires complete excision of the tumor by segmental resection, and should be performed for any patient in whom this can be achieved.34 Due to low rates of lymphatic metastases, lymphadenectomy is not required.28,54 Care must be exercised to maintain the tumor capsule, as pre- or intraoperative tumor rupture is associated with much higher rates of recurrence.19,29,55 Even large GISTs can be completely resected, due to their growth patterns which more often push rather than invade adjacent organs.34 At the time of surgery, a thorough search for metastatic disease is performed (Fig. 104-2).28 Postoperatively, patients with GISTs at moderate to high risk of recurrence should receive 36 months of adjuvant imatinib treatment.29 Recurrence risk assessment for GISTs arising in the small bowel differs from that of gastric GISTs, and moderate to high risk tumors are those with intraperitoneal tumor rupture, those >5 cm, those with >5 mitoses/50 high power fields, or in the setting of an R1 resection.29 Outcomes in small bowel GIST do not correlate well with the traditional TNM staging system, making alternative risk assessments that incorporate mitoses, size, and location preferred.28,29,56
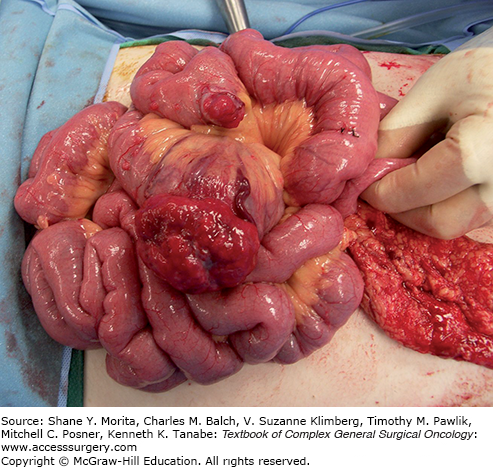
FIGURE 104-2
This patient with neurofibromatosis type 1 presented with abdominal pain, gastrointestinal bleeding, and syncopal episodes due to anemia. Capsule endoscopy showed a lesion in the small bowel, and laparotomy demonstrated a gastrointestinal stromal tumor appearing as a hyperemic mass protruding from the small bowel wall. An additional tumor deposit is evident on an adjacent loop. As is more common in both NF1-associated and small bowel GISTs, this tumor had no detectable KIT mutation.
Although tumors must be completely grossly excised with a goal of R0 resection with microscopically negative margins, the importance of achieving microscopically negative margins in the imatinib era is debatable. A review of patients enrolled in the adjuvant imatinib Z9000 and Z9001 trials revealed that while pre- or intraoperative tumor rupture was associated with significantly worse recurrence-free survival, if ruptured patients were excluded, there was no difference in RFS between patients with R0 and R1 resections (3-year RFS 79% in R1, 80% in R0).55 Thus, residual gross disease should be reexcised, but reexcision is not required in the case of an R1 resection.
Prior to these data, it was hypothesized that neoadjuvant imatinib treatment might improve rates of R0 resection in large or high-risk GISTs, but as the apparent importance of R0 resection has diminished, the focus of neoadjuvant treatment has shifted to shrinking bulky tumors. The RTOG0132 phase II trial tested 12 weeks of neoadjuvant treatment with 600 mg daily imatinib in 30 patients with large primary and 22 patients with metastatic GIST, and established that neoadjuvant imatinib was not associated with excess surgical complications, and that the majority of both groups were able to have R0 resections.24 The EORTC STBSG study reviewed 161 patients with locally advanced GISTs who received neoadjuvant imatinib treatment.21 These patients received a longer course of treatment than in the RTOG trial (median 40 weeks), and tumor shrinkage was noted in 80.1%. The authors concluded that neoadjuvant therapy should be considered when tumor shrinkage might allow preservation of adjacent organs or a less mutilating surgery in locally advanced GIST, that the optimal neoadjuvant treatment duration is likely 4 to 12 months to achieve a maximal response, and that all patients should continue imatinib postoperatively.21 These recommendations are reflected in current National Comprehensive Cancer Network (NCCN) guidelines.29
With the success of imatinib in treating resectable disease, the role of resection in advanced and metastatic GIST is now under investigation. Resistance to imatinib occurs due to emergence of resistant clones with additional activating mutations, usually after 2 to 3 years of treatment.31,33 It is therefore hypothesized that in patients with advanced or metastatic disease who respond to initial therapy, surgical removal of residual disease prior to the emergence of resistant tumor cells could be beneficial. Retrospective results in 69 patients who underwent surgery for metastatic GIST revealed that outcomes strongly correlated with response to imatinib.57 Those with stable disease on imatinib (n = 23) had 1-year overall survival rates of 95%, compared to 86% in those with minimally progressive disease (n = 32), while those who had progressive disease on imatinib prior to surgery all died (n = 14). Other series reported similar findings, but selection and lead-time biases limit the conclusions from these nonrandomized data.32,33,36 In contrast, a study of cytoreductive surgery for patients treated with sunitinib after progression on imatinib found no difference in outcomes based on whether they responded or progressed on sunitinib,58 although this may have been due to low study power. While it seems reasonable to conclude that surgery is not beneficial in patients not responding to imatinib, whether surgery offers an additional benefit in patients who initially respond to or have disease stabilization with imatinib remains unknown. In practice, most surgeons currently resect responsive disease when feasible, and a phase III randomized trial designed to test whether surgery is beneficial for responsive metastatic disease (NCT00956072) was terminated due to poor accrual.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






