Figure 157.1 Pulmonary tuberculosis. Characteristic findings on chest x-ray (CXR) include upper lobe infiltrates, involvement of apical or posterior segments, and cavities with thick walls, smooth inner contours, and no air–fluid levels. Pleural reaction and distal infiltrates from endobronchial spread may be seen. Disease activity cannot be determined from the CXR alone and must be proven or excluded by sputum smear and culture. (Courtesy of David Schlossberg, MD.)
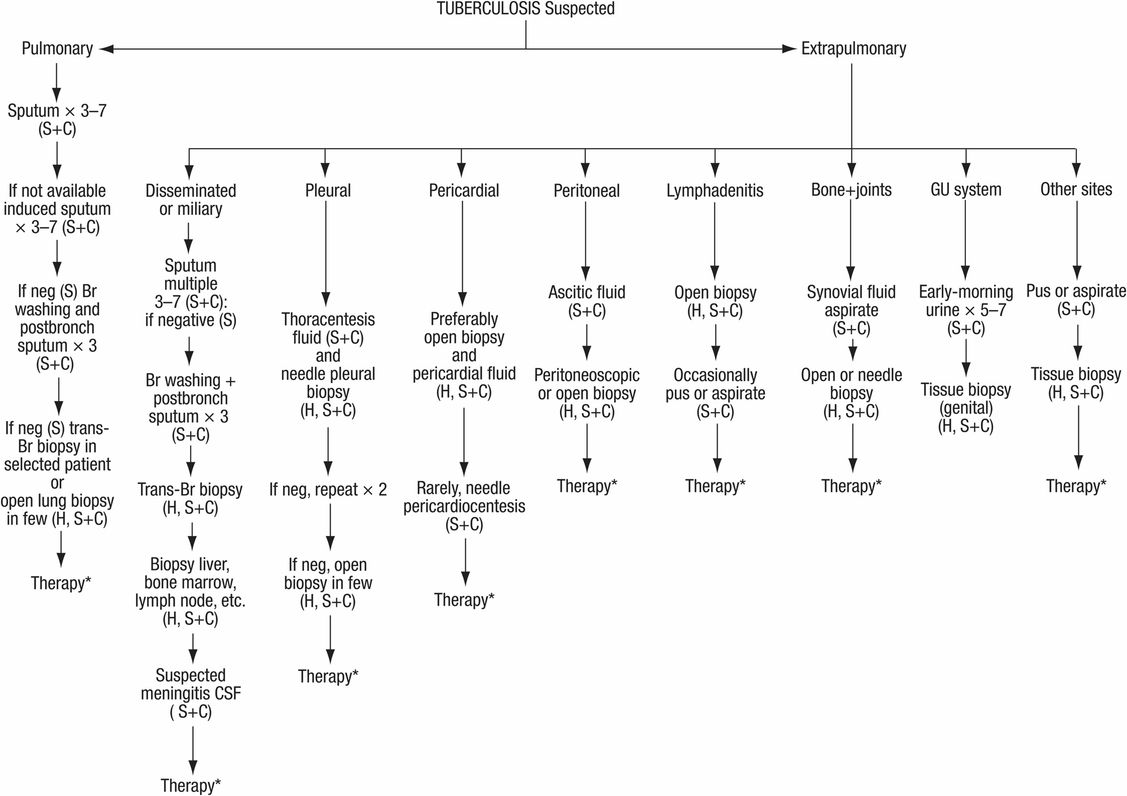
Figure 157.2 Diagnosis of suspected tuberculosis. S = smear; C = culture for mycobacteria; H = histology; Br = bronchial; Bronch = bronchoscopy; CSF = cerebrospinal fluid; neg = negative; GU = genitourinary. * Therapy started in suspected cases, awaiting culture results and/or clinical response.
Latent TB infection
The goal of testing for latent TB infection is to identify the individuals who are at a higher risk for developing TB, and hence who would benefit from preventive therapy. Tuberculosis skin test (TST) has been in use for many decades, but the results are flawed and unreliable in detecting TB infection. With advancement in immunology and genomics, T-cell–based in vitro assays of interferon (IFN) released by T cells after stimulation with M. tuberculosis antigens are developed to identify TB infection. Two interferon-gamma release assays (IGRAs) are available as commercial kits: the Quanti FERON-TB gold assay (QFT-GIT) and the T-SPOT.TB (Oxford Immunotec).
Studies indicate that IFN-γ detection has higher specificity for M. tuberculosis and less cross-reactivity with bacille Calmette–Guérin (BCG) vaccination than TST. The sensitivity of T-SPOT.TB appears to be higher than for QFT-GIT or TST, likely because the testing platform ensures that an adequate number of mononuclear cells are available even if the lymphocyte count is low. The Centers for Disease Control and Prevention (CDC) recommends the QFT-GIT test for detection of latent infection, which has the advantage of a single test for patients. IGRAs should not be used for diagnosis of active TB. The guidelines suggest that this test can be used in place of, but not in addition to TST in situations in which CDC recommends use of TST. Although the evidence is limited, IGRAs appear to be unaffected by NTB infection. Mycobacterium marinum and Mycobacterium kansasii infection are exceptions.
Diagnosis of tuberculosis
Nucleic acid amplification (NAA) tests amplify nucleic acid regions and identify the M. tuberculosis complex. The NAA test can be directly used in clinical specimens (such as sputum) as “direct amplification tests.” The Amplicor MTB test (Roche Diagnostic System), the Amplified Mycobacterium Direct test (MTD) (Gene-Probe, Inc.), and the BD Probe-Tec ET assay (Becton Dickinson Biosciences) are commercially available. However, NAA tests cannot replace conventional tests (microscopy and culture) and should be interpreted along with conventional tests and clinical data.
Rapid detection drug resistance
Line probe assays are novel DNA strip tests that use the polymerase chain reaction (PCR). Commercially available kits include the INNO-LiPA RIF TB kit (Immunogenetics) and Geno Type MTBDR assays (Hain Lifescience). These kits are not US Food and Drug Administration (FDA) approved. Although sensitivity on culture isolates may be over 95% in detecting rifampin resistance, the tests are expensive and require sophisticated laboratory support.
Also, phage-based assays are available as commercial kits but are not approved by the FDA. The test is performed in culture isolates and has high sensitivity but low specificity. This may be used for rifampin resistance in culture isolates, which increases turnaround time. The tests show promise but are not routinely used.
Extrapulmonary tuberculosis
Extrapulmonary TB cases represent 15% to 20% of the total cases but this percentage could be higher in those with HIV coinfection. TB can involve any organ of the body but the lymphatic system and bone are common sites. Miliary and central nervous system TB are rare but carry high morbidity and mortality. In most extrapulmonary TB cases plain radiograph is frequently not adequate to prove the diagnosis. CT scan or MRI is required.
Signs and symptoms of extrapulmonary TB depend upon the site involved. Atypical presentation such as chronic pain, fatigue, and failure to thrive are not uncommon in elderly patients. Clinical presentation of miliary TB can be acute or subacute. Multiorgan failure including acute respiratory distress syndrome can be life threatening. Tissue diagnosis by microscopy and culture or DNA probe confirmation is required if secretions are negative for acid-fast bacilli smear and culture. Frequently treatment has to be started while the final bacteriologic confirmation is pending.
Patients with lymphatic TB generally present with pain and swelling in the area of involvement. In children cervical lymph nodes are frequently involved.
Bone and joint disease may present with joint pain or back ache (Pott’s disease). With chronic disease, destruction of bone with local sclerosis and spinal deformity is noted. Disease is most common in the lower thoracic and lumbar vertebrae. Involvement of the surrounding soft tissue may lead to cold abscess.
For the diagnosis of extrapulmonary TB, secretions and/or biopsy material must be obtained from the site (Figure 157.2). In the case of tuberculous meningitis it may be necessary to initiate therapy empirically because the disease may become irreversible before the diagnosis can be made. Low glucose, high protein, and lymphocytosis is frequently noted in cerebrospinal examination.
Therapy
Principles of chemotherapy
Initial treatment of TB should include four drugs: generally isoniazid (INH), rifampin (RIF), ethambutol (EMB), and pyrazinamide (PZA). Directly observed therapy (DOT) is the preferred strategy. After daily treatment for the first 4 to 6 weeks, a twice or three time weekly regimen can be selected. Table 157.1 lists drugs, dosages, and major side effects. Several first-line bactericidal drugs are commonly combined initially because they reduce the bacterial population rapidly without the risk of resistance. Second-line drugs are most useful when resistance to two or more first-line drugs is found or they cannot be used because of life-threatening side effects or intolerance (Figure 157.3). Currently 11 drugs are approved by the FDA. Of the approved drugs, INH, RIF, EMB, and PZA are considered first-line drugs. While fluoroquinolones are not FDA approved for TB, they are commonly used for drug-resistant TB cases. Rifabutin (RBT) and rifapentine (RPT) can be considered as important drugs in certain circumstances. Streptomycin (SM) is no longer included in the list of first-line drugs.
| Drug | Daily dosage | Twice-weekly dosage | Side effects | Mode of action |
|---|---|---|---|---|
| First-line drugs | ||||
| Isoniazid (INH) | 5 mg/kg (usually 300 mg) PO or IM | 15 mg/kg (usually 900 mg) PO | Peripheral neuritis, hepatotoxicity, allergic fever and rash, lupus erythematosus phenomenon | Acts strongly on rapidly dividing extracellular bacilli; acts weakly on slowly multiplying intracellular bacilli |
| Rifampin (RIF) | 10 mg/kg (usually 450–600 mg) PO | 10 mg/kg (usually 450–600 mg) PO | Hepatotoxicity, nausea, vomiting, allergic fever and rash, flu-like syndrome, petechiae with thrombocytopenia or acute renal failure during intermittent therapy | Acts on both rapidly and slowly multiplying extracellular and intracellular bacilli, particularly on slowly multiplying persisters |
| Rifabutin (Ansamycin) | 300 mg PO daily, twice or thrice weekly | Used as RIF substitute | Same as rifampin; uveitis, arthralgia, leukopenia | Same as above |
| Rifapentine | 300–600 mg PO once weekly in continuation phase | Seronegative HIV, noncavitary TB | Same as rifampin; not used in HIV | |
| Rifamate (INH 150 mg plus RIF 300 mg) | 2 capsules PO qd | 2 capsules plus 2 tablets of INH (300 mg) | Same as INH/RIF | Same as INH/RIF |
| Rifater (INH 50 mg plus RIF 120 mg plus PZA 300 mg) | 5–6 capsules PO qd | Same as INH/RIF/PZA | Same as INH/RIF/PZA | |
| Pyrazinamide (PZA) | 25–30 mg/kg PO (usually 1.5–2 g) | 45–50 mg/kg PO (usually 3–3.5 mg) | Hyperuricemia, hepatotoxicity, allergic fever and rash | Active in acid pH (2.5 g) on intracellular bacilli |
| Ethambutol (EMB) | 15–25 mg/kg/d initially, followed after 2 months with 15 mg/kg/d | 50 mg/kg PO | Optic neuritis, skin rash, hyperuricemia | Weakly active against both extracellular and intracellular bacilli to inhibit the development of resistance |
| Second-line drugs | ||||
| Streptomycin | 10–15 mg/kg (usually 0.5–1 g) 5 days/week; IM or IV | 20–25 mg/kg (usually 1–1.5 g) | Cranial nerve VIII damage (vestibular and auditory), nephrotoxicity, allergic fever, rash | Active against rapidly multiplying bacilli in neutral or slightly alkaline extracellular medium |
| Kanamycin | 15–30 mg/kg qd IM or IV | 15–30 mg/kg | Same as streptomycin | Same as streptomycin |
| Amikacin | 15–30 mg/kg qd IM or IV | 15–30 mg/kg | Same as streptomycin | Same as streptomycin |
| Capreomycin | 15–30 mg/kg/d IM or IV | 15–30 mg/kg | Same as streptomycin | Same as streptomycin |
| Ethionamide | 10–15 mg/kg (usually 500–750 mg) in divided doses PO with 100 mg pyridoxine | Not used | Nausea, vomiting, anorexia, allergic fever and rash, hepatotoxicity, neurotoxicity, hypothyroidism | Same as streptomycin |
| Cycloserine | 15–20 mg/kg (usually 0.75–1 g) in divided doses with 200 mg pyridoxine PO | Not used | Personality changes, psychosis, convulsions, rash | Same as ethambutol |
| Para-aminosalicylic acid | 150 mg/kg (usually 12 g) in divided doses PO | Not used | Nausea, vomiting, diarrhea, hepatotoxicity, allergic rash and fever, hypothyroidism | Weak action on extracellular bacilli; inhibits development of drug-resistant organisms |
| Thiocetazonea | 150 mg PO | Used rarely | Allergic rash and fever, Stevens–Johnson syndrome, blood disorders, nausea, vomiting | Same as para-aminosalicylic acid |
| Clofazimine | 200–300 mg PO qd | Not used | Pigmentation of skin, abdominal pain | Not fully known |
| Newer agents | ||||
| Ofloxacin | 400 mg q12h | Not used | Gastrointestinal: diarrhea, nausea, abdominal pain, anorexia; central nervous system: dizziness, restlessness, nightmares, ataxia, seizures | Rapidly multiplying bacilli at neutral or alkaline pH |
| Gatifloxacin | 400 mg PO qd | Not used | Same as ofloxacin | Same as ofloxacin |
| Levofloxacin | 500 mg PO qd | Not used | Same as ofloxacin | Same as ofloxacin |






