In the United States, rectal cancer is diagnosed in approximately 40,000 individuals annually.1 Although definitions vary, locally advanced rectal cancer generally refers to patients with T3/T4 disease and/or positive lymph nodes.2 In patients with locally advanced rectal cancer, treatment with surgery alone results in substantial risk of local recurrence; therefore, multimodality therapy is recommended.
Common clinical symptoms of rectal cancer include a change in stool caliber, hematochezia, rectal pain, tenesmus, or obstructive symptoms (abdominal pain, nausea/vomiting, obstipation). The presence of specific symptoms may aid in diagnosis: tenesmus is generally associated with bulky or fixed tumors, while pain with defecation suggests involvement of the distal one-third of the rectum. On history, full evaluation of rectal function, urinary function, and sexual function is crucial, not only for diagnosis but also to predict the extent of tumor involvement and to plan operative therapy. On physical examination, digital rectal examination is used to assess the position of the tumor (relative to the anorectal ring) and fixation to the anal sphincters, the levators, and the bony pelvis. Digital rectal examination can also assess the degree of obstruction for distal tumors. Female genital tract involvement can be evaluated by completing a rectovaginal examination.
Following history and physical examination, a colonoscopy is generally indicated to further workup symptoms suspicious for rectal cancer. Colonoscopy is the gold standard for diagnosis of rectal cancer because it allows for tissue diagnosis of the primary lesion, evaluation of the remainder of the colon for synchronous tumors (the incidence of synchronous tumors is approximately 4%), and removal of any polyps.3 In addition to full colonoscopy, rigid sigmoidoscopy can be used to determine the position of the tumor within the rectum (anterior, posterior, left, or right) and the distance between the distal tumor margin and the anal verge. Following tissue diagnosis, patients require staging for locoregional and metastatic disease.
The goals of locoregional staging for rectal cancer are to determine the optimal surgical approach and to determine whether neoadjuvant therapy is required. Locoregional staging utilizes imaging to assess for the depth of tumor invasion, the presence of lymph node involvement, invasion into adjacent organs, and to determine the circumferential radial margin (CRM). Although some information can be gained from computerized tomography (CT) scan, magnetic resonance imaging (MRI) and transrectal ultrasound (TRUS) are the most helpful imaging modalities for locoregional staging of rectal cancer. All patients with locally advanced rectal cancer should undergo staging with CT scan of the chest, abdomen, and pelvis along with evaluation of the rectal tumor with either MRI or TRUS.
Compared to MRI and TRUS, CT scan has decreased sensitivity and specificity for identifying T-stage, N-stage, and CRM for rectal cancer.4 CT scan can, however, be useful in predicting adjacent organ involvement and in identifying tumor-related complications of rectal cancer including obstruction, perforation, or fistula formation.5 In addition, CT scan is accurate in assessing for metastatic disease (see “Staging for Metastatic Disease” below).
Thin-cut MRI with phased array coil MRI is the preferred imaging modality for the assessment of the locoregional involvement of locally advanced rectal cancer. MRI is very accurate for prediction of CRM and T-stage in rectal cancer, but is limited in the assessment of lymph node involvement. In a recent meta-analysis assessing the diagnostic accuracy of MRI for rectal cancer, the sensitivity and specificity for predicting T-stage were 87% and 75%, respectively; the sensitivity and specificity for predicting CRM were 77% and 94%, respectively; while the sensitivity and specificity for identifying positive lymph nodes were 77% and 71%, respectively.6 MRI is also very accurate in assessing for tumor infiltration of surrounding structures: in one study, MRI has a sensitivity of up to 97% and a specificity of 98% in the prediction of tumor infiltration of surrounding structures.4
While TRUS is also useful in the locoregional staging of rectal cancer, it is most beneficial in the locoregional staging of patients with early-stage disease. TRUS is particularly helpful in distinguishing tumor involvement beyond the muscularis propria (T1/T2 lesions vs. T3 tumors).7 The accuracy of TRUS in determining lymph node involvement is similar to that of MRI; both TRUS and MRI are superior to CT in determining lymph node involvement.8 TRUS is inferior to MRI in assessing tumor involvement of the CRM. To accurately perform TRUS, the ultrasound probe must be passed through the tumor; thus, the role of TRUS is limited in patients with bulky, stenotic, or high rectal tumors.
The National Comprehensive Cancer Network (NCCN) recommends that all patients with rectal cancer undergo staging for metastatic disease with carcinoembryonic antigen (CEA) and with CT of chest, abdomen, and pelvis.9 CT scan of the abdomen and pelvis should be performed with oral and IV contrast. For patients in whom IV contrast is contraindicated, an abdominal/pelvic MRI with contrast and a noncontrast CT chest should be performed. At this point, there is no evidence to support routine use of positron emission tomography (PET) or PET/CT in staging for rectal cancer.
Patients with locally advanced rectal cancer are at risk for local recurrence in addition to distant metastases.10,13 Rectal cancer is particularly prone to local recurrence due to the absence of serosa surrounding the rectum, the proximity of the rectum to pelvic structures and other organs, and technical difficulties in obtaining wide circumferential margins in the pelvis. Accordingly, for the majority of patients with locally advanced rectal cancer (stage II and stage III), combined modality therapy consisting of (1) neoadjuvant chemoradiation, (2) surgery, and (3) adjuvant chemotherapy is recommended to decrease the risk of both local and distant recurrence. All patients with locally advanced rectal cancer should undergo preoperative consultation with medical oncology, radiation oncology, and the extirpative surgeon and cases should be presented at multidisciplinary cancer conference to determine the best course of treatment for each patient.
Up to 30% of patients with colorectal cancer present with acute intestinal obstruction.11 Most patients who present with obstructed rectal cancer have T3 or T4 disease, and thus neoadjuvant chemoradiotherapy is preferred prior to tumor resection. In this situation, both rectal stenting and emergency diversion have been used as a bridge to neoadjuvant chemoradiotherapy and subsequent extirpative surgery. Stenting of rectal tumors is frequently associated with pain and stent migration; consequently, fecal diversion is the recommended approach in patients who present with acute obstruction.12 Our preferred approach is a laparoscopic loop sigmoid colostomy (if there is sufficient colonic length) in patients who will require a permanent stoma and a laparoscopic loop ileostomy in patients in whom a sphincter-preserving procedure is planned. Patients with a competent ileocecal valve and a complete obstruction may require proximal transverse colostomy. Patients who present with partial obstruction, in our experience, will tolerate neoadjuvant therapy without fecal diversion if a pediatric esphagogastroduodenal scope can be passed beyond the tumor.
Historically, radiation for rectal cancer has been delivered both alone and in conjunction with chemotherapy and has been administered in both the neoadjuvant and the adjuvant setting. The goals of neoadjuvant treatment for rectal cancer are to (1) decrease local recurrence, (2) downstage the tumor, (3) improve sphincter salvage, and (4) decrease the toxicity of radiotherapy (compared to adjuvant treatment).
Neoadjuvant chemoradiotherapy is considered the standard of care for patients with T3 and T4 rectal cancer based on the results of randomized controlled trials. Additional relative indications for neoadjuvant chemoradiotherapy include node positivity on MRI or TRUS in a patient with T1/T2 rectal cancer, distal tumors for which an abdominoperineal resection (APR) is thought to be required and tumors in which the circumferential margin is threatened.
The German rectal cancer established neoadjuvant chemoradiotherapy as the standard of care for patients with T3/T4 or node-positive rectal cancer.13 In this trial, 823 patients were randomized to preoperative chemoradiotherapy (total of 5040 CGy in 28 fractions plus infusional fluorouracil given during the first and fifth weeks of treatment) or postoperative chemoradiotherapy (the same treatment with the addition of a 540-cGy boost to the tumor bed). All patients in this study underwent total mesorectal excision (TME) (6 weeks after conclusion of chemoradiation for patients in the preoperative treatment arm) and also received four cycles of bolus adjuvant fluorouracil. In this trial, the incidence of local recurrence was significantly decreased in the patients assigned to preoperative chemoradiotherapy (5-year local recurrence 6% vs. 13%, p = 0.006). There was no difference in overall survival comparing patients assigned to preoperative treatment to those assigned to postoperative treatment (5-year overall survival 76% vs. 74%, p = 0.80). In addition, patients who received preoperative chemoradiotherapy had significant downstaging, improved sphincter salvage, and decreased acute and long-term toxic effects, compared to patients randomized to postoperative chemoradiotherapy.
Neoadjuvant chemoradiotherapy has been compared to neoadjuvant radiotherapy alone in several trials. A recent meta-analysis of six randomized controlled trials comparing neoadjuvant chemoradiotherapy to neoadjuvant radiotherapy in patients with T3/T4 rectal cancer demonstrated decreased local recurrence in patients receiving neoadjuvant chemoradiotherapy.14 There was no difference in overall survival or sphincter preservation; however, increased acute toxicity was seen in the chemoradiotherapy group.
A number of randomized controlled trials have been completed assessing various chemotherapy regimens used during neoadjuvant radiotherapy. The current standard of care is either infusional 5-FU or oral capecitabine.15 Multiple randomized controlled trials have demonstrated increased toxicity and no improvement in pathologic complete response rate, sphincter salvage, or overall survival with the addition of neoadjuvant oxaliplatin to long-course chemoradiotherapy with infusional 5-FU.16–19 Similarly, the addition of neoadjuvant irinotecan to chemoradiotherapy has not been associated with improvements in overall survival, disease-specific survival, or pathologic complete response rates.20
The optimal timing of surgery following long-course chemoradiotherapy has not been established. Traditionally, this interval has been 6 weeks, based on the German Rectal Cancer Trial.13 Because tumor regression takes time, it has been suggested that waiting a longer period between the conclusion of chemoradiation and surgery may result in higher pathologic complete response rates and better outcomes. A meta-analysis of 13 uncontrolled trials demonstrated that waiting beyond 8 weeks after the conclusion of radiotherapy results in a 6% increase in pathologic complete response rate; however, morbidity, sphincter preservation, and oncologic outcomes (overall survival, disease-free survival, R0 resection rate) were not improved with a longer waiting time.21 The clinical significance of an improved pathologic complete response rate in the absence of improved oncologic outcomes is debatable. Our practice is to wait 8 to 12 weeks between neoadjuvant therapy and surgery.
Short-course preoperative radiation represents an alternative to traditional long-course chemoradiotherapy. Short-course radiotherapy (25 Gy in 5 fractions) was compared to surgery alone in the Swedish Rectal Cancer Trial.22 In this trial, patients underwent surgery 1 week following completion of radiotherapy. In patients who underwent preoperative radiation, both local recurrence (5-year local recurrence 11% vs. 27%, p < 0.001) and overall survival (5-year overall survival 58% vs. 48%, p = 0.004) were improved compared to patients who underwent surgery alone. This is the only trial that has demonstrated a survival benefit to neoadjuvant radiotherapy. The Dutch rectal cancer trial compared short-course neoadjuvant radiotherapy with TME to TME alone.23,24 In the Dutch trial, patients undergoing short-course neoadjuvant radiotherapy had improved local recurrence (5-year local recurrence 5.6% vs. 10.9%, p < 0.001) compared to those undergoing TME alone; however, overall survival was not different (5-year overall survival 64.2% vs. 63.5%, p = 0.902) between the two groups. Two randomized controlled trials have compared short-course radiation to long-course chemoradiation. Both of these trials demonstrated improved pathologic complete response rates with long-course chemoradiotherapy. There were no differences seen in recurrence-free or overall survival comparing short-course radiotherapy to long-course chemoradiotherapy in these trials; however, both of these trials were underpowered to demonstrate equivalence of these treatments.25,26 In North America, long-term chemoradiotherapy is generally the preferred approach. In patients who present with metastatic disease, short-course radiotherapy is occasionally used to prevent delays in initiation of systemic therapy.
Following the German Rectal Cancer Trial, which established neoadjuvant chemoradiotherapy as the standard of care, indications for adjuvant chemoradiation or radiation are limited. For patients with resected stage II and III rectal cancer who do not undergo neoadjuvant therapy, postoperative chemoradiotherapy is preferable to surgery alone.
A number of older clinical trials examined outcomes with adjuvant chemoradiotherapy or radiotherapy. A large meta-analysis including seven trials comparing surgery alone to surgery followed by radiotherapy demonstrated that adjuvant radiotherapy was associated with decreased local recurrence (5-year local recurrence 15.3% vs. 22.9%, p < 0.001), but did not result in improved overall survival.27 In addition, two randomized controlled trials comparing adjuvant chemoradiotherapy (using older chemotherapy regimens) to radiation alone demonstrated decreased local recurrence and improved survival.28–30
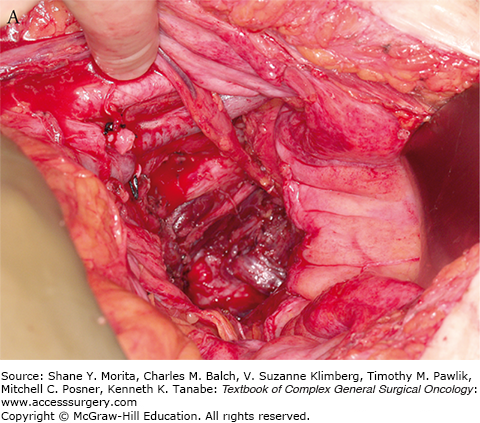
Intraoperative radiotherapy (IORT) has been used in conjunction with neoadjuvant chemoradiotherapy in a number of centers in the United States, Europe, and Asia, with the goal of further decreasing the risk of local recurrence in patients with locally advanced rectal cancer. IORT delivers a boost of radiotherapy to the operative bed at the time of surgery. The goal of IORT is to sterilize any remaining microscopic foci of tumor following surgical resection (Fig. 113-1A). The benefit of IORT on locoregional recurrence is thought to be additive to the benefit of neoadjuvant chemoradiotherapy.
FIGURE 113-1
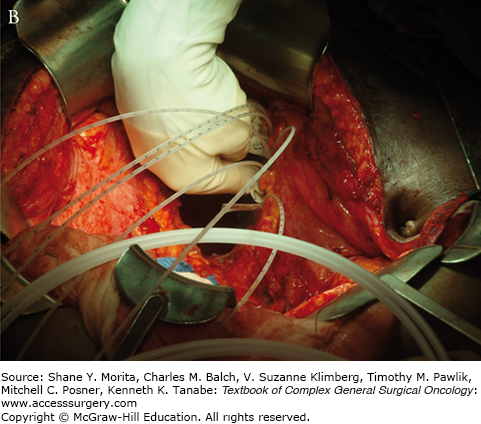
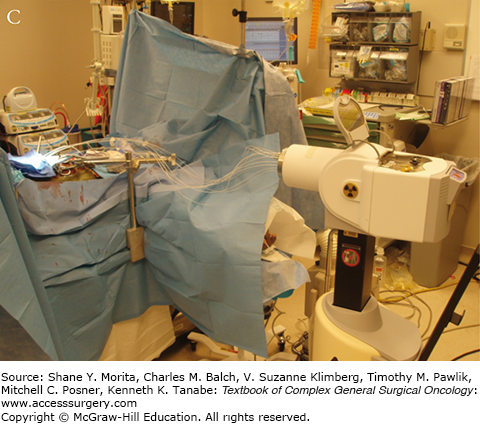
Setup and delivery of intraoperative electron beam radiation therapy (IOERT): A. The operative field of a patient following resection of locally advanced rectal cancer abutting the sciatic notch and the left pelvic sidewall. The internal iliac artery and vein have been ligated and the tumor has been dissected off of the left pelvic sidewall. B. Intraoperative setup for IOERT. Cone is used to deliver focused intraoperative electron beam radiation therapy to the operative bed. C. Delivery of IOERT using a mobile linear accelerator.
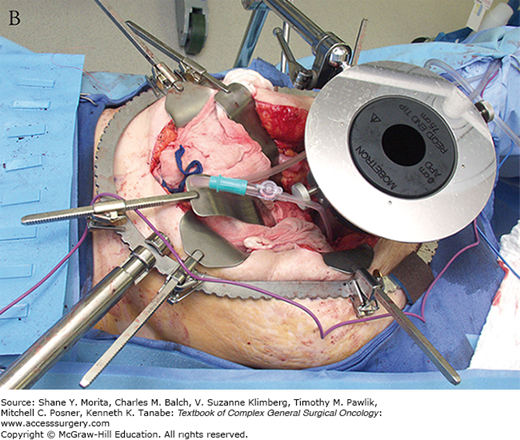
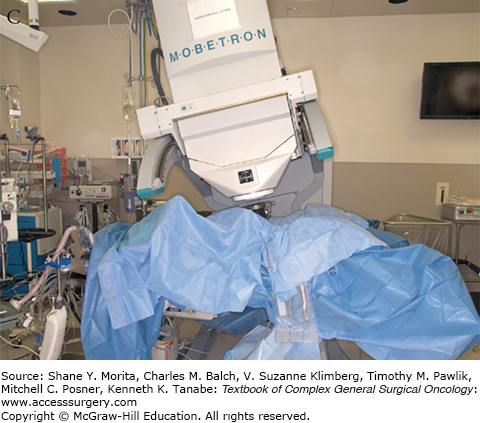
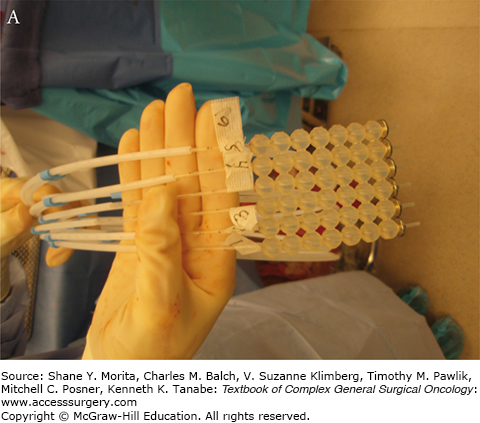
Two types of IORT are commonly used in the treatment of locally advanced rectal cancer: intraoperative electron beam radiation therapy (IOERT) and high-dose rate intraoperative radiation therapy (HDR-IORT).31 IOERT is delivered using electrons using a fixed or mobile linear accelerator. IOERT can be delivered in any operating room (there is no need for a lead shielded operating room), because electrons are used which do not penetrate the tissue as deeply as conventional radiotherapy. Multiple cone shapes and sizes can be adapted to fit differing tumor bed volumes with IOERT (Fig. 113-1B, C). One limitation of IOERT is that overlapping cones may result in radiation “hot spots” with increased toxicity, particularly peripheral neuropathy. In contrast, HDR-IORT is delivered using high-dose rate brachytherapy administered by catheters placed in the tumor bed following en bloc resection of the tumor (Fig. 113-2A). Catheters are placed into a silicon flap that can be cut to fit the tumor bed (Fig. 113-2B). The entire dose of HDR-IORT is administered at the time of surgery via a wire with an iridium source at the end that passes through the catheters (Fig. 113-2C). In contrast to IOERT which often requires multiple applications to cover the treatment area, the full dose of HDR-RT can generally be administered with a single flap. HDR-RT also results in a higher surface dose and a lower dose at 2 cm than IOERT.
One randomized controlled trial has compared surgery alone to surgery plus IORT in patients with stage II and III rectal cancer who had received neoadjuvant chemoradiotherapy.32 This trial did not demonstrate any difference in local recurrence or overall survival comparing patients who received IORT plus surgery to those who received surgery alone, although the trial was significantly underpowered. Observational studies have demonstrated conflicting results with respect to the efficacy of IORT: some studies have noted decreased local recurrence with IORT,33–37 while other studies have noted no benefit of IORT.38,39 A recent observational study reported that patients with microscopically involved circumferential resection margins who were treated with IORT appear to have significantly improved local recurrence-free survival (5-year local recurrence-free survival 84% vs. 41%, p = 0.01) when compared to patients treated with surgery alone.33 In this study, there was no significant improvement in local recurrence-free survival seen with IORT among patients who underwent an R0 resection. We recommend having IORT available intraoperatively for all patients who undergo surgery for locally advanced rectal cancer with close lateral margins.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree