Year [ref.]
Design
Subjects
Measurements
Factors identified
Odds ratio/subhazard ratio (95 % CI)
2012
[63]
Cross-sectional study
179 postmenopausal women
Bone turnover markers
DXA
Femur structure
Fractal analysis
Prior fracture
25-OHD < 20 ng/ml
Fracture loada × 100 U
OR 3.89 (1.47–8.82)
OR 3.89 (1.55–9.77)
OR 0.96 (0.93–0.99)
2014
[64]
Prospective cohort study (GLOW)
26,918 women, 5550 on treatment. Follow-up 3 years
Yearly questionnaire (self-administered)
SF-36 vitality × 10 points
2 or more fallsb
Prior fracture
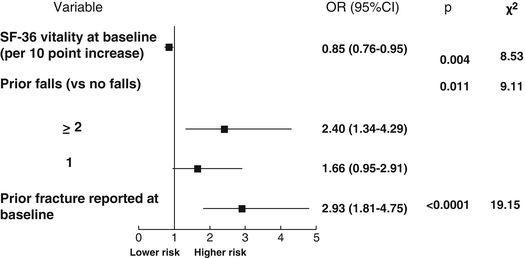
OR 0.85 (0.76–0.95)
OR 2.40 (1.34–4.29)
OR 2.93 (1.81–4.75)
2014
[65]
Nationwide health database (retrospective cohort)
5 million, 21,385 treated with BP
GP e-records + hospital admissions (ICD fracture codes), specialists referrals, pharmacy invoicing (BP (bisphosponates) use, MPR)
Age > 80 vs. 60
Previous fracture
Underweight
Inflammatory arthritis
PPIs use
Vitamin D deficiency
SHR 2.18 (1.70–2.80)
SHR 1.75 (1.39–1.20)
SHR 2.11 (1.14–3.92)
SHR 1.46 (1.02–2.10)
SHR 1.22 (1.02–1.46)
SHR 2.69 (1.27–5.72)
A second opportunity for assessing predictive elements of treatment failure was offered by the GLOW stud y [64]. This is a prospective cohort study of more than 60,000 women from ten countries recruited from 723 primary care practices in 17 sites. These centers were located in six European countries, Canada, the USA, and Australia. Women received a self-administered posted questionnaire including personal characteristics, diagnostics, and treatments received, quality of life, and fracture history. The results of this analysis were those from baseline to the end of the third year of observation. According to the definition of the IOF working group [62], treatment failure was defined as the occurrence of two incident fractures after being on therapy for at least 1 year. During the 3-year study period, 6.5 % of women on continuous therapy with the same drug sustained one fracture, while 1.3 % suffered two or more. The variables associated with treatment failure (≥2 incident fractures) in univariate analyses were a lower score in the SF-36 scale (physical function and vitality) at baseline, higher FRAX© score, falls in the past 12 months, having more comorbid conditions, prior fracture, current use of glucocorticoids, need of arms to assist when standing, and unexplained weight loss. After multivariate analysis, a worse SF-36 vitality score, prior fracture, and two or more falls in the previous year remained as independent predictors. These results are summarized in Table 5.1 and Fig. 5.1.
A third recent investigation has been carried out using the SIDIAP database in Catalonia, Spain [65]. SIDIAP gathers clinical information from electronic records of primary care health providers, prescription and pharmacy invoices, laboratory tests, and hospital admissions. The ICD-10 coding system is used, and the database includes data of more than five million people (over 80 % of the population of Catalonia). The cases analyzed were those patients suffering incident fractures in spite of being persistent for at least 6 months and with a medication possession ratio of 80 % or above. Only 35 % of patients among the 21,385 got a prescription completed at least 6 months of treatment. In this subgroup of persistent individuals, the incidence of fractures while on treatment was of 3.4 per 100 person-years. The predictors of these fractures were older age, previous fracture, underweight, inflammatory arthritis, use of proton pump inhibitors, and vitamin D deficiency (Table 5.1). To note that when starting bisphosphonates, the time elapsed between a prior fracture and the date of treatment initiation was relevant in these data. When the fracture was recent (less than 6 months before starting therapy), the risk was higher than for “older” fractures (those that had occurred more than 6 months before starting the drugs) (Fig. 5.2).
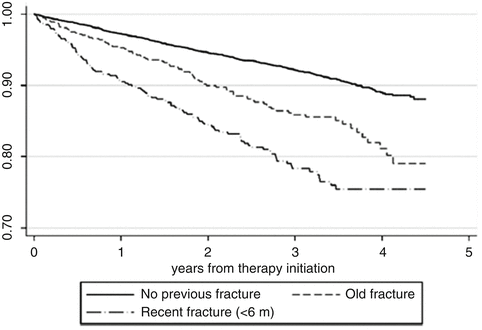

Fig. 5.2
Cumulative incidence of fracture in patients taking bisphosphonates in the SIDIAP database. Kaplan–Meier estimates of cumulative incidence of fracture while on treatment among incident users of bisphosphonates for patients with no previous fracture, recent fracture (in the past 6 months), and an old fracture (more than 6 months prior to therapy initiation)
Concluding Remarks
Patients on treatment with the drugs used in osteoporosis still are at risk of suffering fractures, and the rate observed is around 2–3 % per year. However, not every incident fracture while on treatment indicates a failure of the drug. When a second incident fracture occurs or when, in addition to one fracture, surrogate markers (BMD and/or bone turnover markers) also show a negative evolution, the likelihood for that particular individual of being a nonresponder is high.
A very appealing concept, treatment-to-target, is currently being developed [66]. In fact, the treatment-to-target strategy is symmetrical to treatment failure since it aims to establish when a treatment has obtained a desired effect, that is, the cure or control of the disease. Both together promise to be of great help to the clinician in making decisions on when a treatment can be stopped (target reached) or has to be modified (treatment failed). Again, the available evidence is terribly scarce, and the best clinical judgment in the evaluation of an individual patient is, as of today, the only way to decide when the target has been attained.
Several clinical variables can predict this incident fracture in spite of receiving an active drug with good compliance, in the vast majority of cases for oral bisphosphonates. In general, predictors of fracture while on treatment can be grouped in (1) elements showing an advanced stage of the disease, (2) with a degree of deterioration in bone strength too important to be compensated by the drug effects, or (3) in extra-skeletal elements that can jeopardize the risk reduction obtained by a given drug in the clinical trials. Often, both groups of elements merge in the same individual, and perhaps the most common example is the frail elderly, with several concomitant diseases and taking a number of medications, with deficiency in vitamin D, sarcopenia, and frequent falls potentiated by sedatives, anti hypertensive drugs, and taking regularly proton pump inhibitors to make a multi-pill regime tolerable.
Besides maximizing the effectiveness of currently available therapies by improving treatment adherence and avoiding, where possible, the previously described risk factors (e.g., ensuring vitamin D repletion concomitant or previous to anti-osteoporosis therapy), specific strategies to overcome the therapeutic ceiling of these medications, by developing new molecules or combining the ones we have today, are advisable. Parenteral administration can represent a progress for ensuring full adherence and bioavailability of drugs that have no need to be absorbed. But, with no doubt, medical care has to be more comprehensive than merely prescribing a good drug for bones. Other health problems and medications should be reviewed, and rehabilitation, psychosocial support, and integral care are key for extracting from our drugs the maximal benefit.
References1
1.
Bloch-Michel H, Milhaud G, Coutris G, Waltzing P, Charret A, Morin Y, et al. [Long term treatment of osteoporosis with thyrocalcitonin. Apropos of 7 cases]. Revue du rhumatisme et des maladies osteo-articulaires. 1970;37(10):629–38.PubMed
2.
Dambacher MA, Olah AJ, Guncaga J, Lentner C, Haas HG. [Metabolic rates and histological-morphometrical investigations in bones of patients with osteoporosis subjected to long-term application of calcitonin]. Acta endocrinologica Supplementum. 1971;152:87.PubMed
3.
4.
Wallach S, Aloia J, Cohn S. Treatment of osteoporosis with calcitonin. Semin Drug Treat. 1972;2(1):21–5.PubMed
5.
6.
*Dowd R, Recker RR, Heaney RP. Study subjects and ordinary patients. Osteoporos Int. 2000;11(6):533–6. *This paper shows how ordinary patients in everyday’s practice do not fulfill inclusion criteria or have major exclusion criteria when compared with the cases included in the pivotal trials for osteoporosis treatment.PubMedCrossRef
7.
8.
9.
*Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81(8):1013–22. *When adherence to treatment decreases, so does the antifracture efficacy. Below 80% adherence efficacy declines and for adherence rates of 50% & or less, the treatment efficacy totally vanishes.PubMedCrossRef
10.
11.
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11(1):83–91.PubMedCrossRef
12.
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282(14):1344–52.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree