Epidemiology and risk factors
Toxoplasma gondii is a protozoan parasite in the phylum Apicomplexa. It infects humans and almost all warm-blooded animals, including mammals and birds.
Transmission of T. gondii is linked to its biological life cycle and occurs most frequently with the ingestion of oocysts in soil and water or with the ingestion of tissue cysts from infected animals and, less frequently, with transplacental dissemination to a fetus ( Fig. 29.1 ). Tissue cysts can be found in any organ, so organ transplantation is also a potential source of transmission ( Table 29.1 ). The Felidae family, domestic and stray cats and wild felids (e.g., cougars, bobcats, lions), are the definitive host in which T. gondii undergoes sexual reproduction (see Fig. 29.1 ). Infected cats can shed more than 100 million oocysts in their stool. Unsporulated oocysts in the environment are not infectious until matured, which takes 1 to 5 days; therefore direct contact with cats is not considered a transmission risk. Sporulated oocysts remain viable for more than a year in moist environments because they can resist chemical and mechanical damage and detergents.

| Risk Level | Type of Patient (Mode of Transmission) (Incidence) |
|---|---|
| High | HSCT (reactivation) (2.1%-19.6%) D+/R− Heart transplant (graft transmission) (0.6%-25%) |
| Intermediate | D + /R − Liver, kidney, lung, small intestine, pancreas (graft transmission) (0.08%-0.2%) Enhanced immunosuppression in HSCT, SOT, oncology patients (e.g., with treatment for GVHD or allograft rejection) |
| Low | Seropositive R + SOT recipients (reactivation) Seropositive oncology patients (reactivation) Autologous HSCT (reactivation) |
| None | Eye, bone, artery transplant |
Oocysts house a protozoan stage of T. gondii called sporozoites. If a human or animal ingests oocysts, the gastric enzymes destroy the cyst wall and sporozoites penetrate the intestinal epithelium and differentiate into another stage (rapidly dividing tachyzoites). Tachyzoites can invade almost all nucleated cells and remain intracellular, evading the immune response within a parasitophorous vacuole.
Tachyzoites disseminate throughout the body via the blood or lymph circulation, transported within a monocyte, dendritic cell, or other nucleated cells, and can lodge in any tissue, including a fetus via a transplacental route. Tachyzoite replication is generally thought to be controlled by the host immune response within a few weeks after initial infection. Outside the host cell, tachyzoites are fragile and are usually killed rapidly. Theoretically, during the acute dissemination phase, tachyzoites could be transmitted to a patient if the bone marrow or blood was collected during this transient phase. Reports have suggested this scenario but have not been confirmed.
Within the tissue, asexually replicating tachyzoites can differentiate into bradyzoites, a slowly dividing stage that eventually forms a tissue cyst. The signals for differentiation between the stages are not completely defined. Tissue cysts can develop as early as 7 to 10 days after initial infection and can form in any organ. More often, tissue cysts are found in neural tissues, such as the brain and the eye, and in muscular tissues such as skeletal muscles and the heart. Tissue cysts can remain in the host for years.
Protective immunity may be sustained throughout the lifetime because of antigenic stimulation from intermittent asymptomatic reactivation of persistent tissue cysts. The host immune response is protective but does not eradicate the protozoa, resulting in a balance that allows survival of the host by limiting T. gondii replication and allows persistence of the protozoa by subverting the host immune response. If tissue cysts rupture in an immunocompromised individual, bradyzoites transform into tachyzoites that can disseminate to any tissue, transforming back into bradyzoites and forming new tissue cysts. “Reactivation” of persistent tissue cysts can lead to uncontrolled tachyzoite replication and dissemination, leading to the clinical signs and symptoms observed in severely immunosuppressed patients. Interferon-gamma–secreting CD4 and CD8 T cells and natural killer (NK) cells are important for controlling bradyzoite reactivation within a cyst, because interferon-gamma is key for controlling acute T. gondii infection. Therefore immune suppression medications that interfere with cell-mediated immunity or myeloablative conditioning can increase the risk of T. gondii infection and disease. The level of immune suppression also contributes to the clinical severity of T. gondii disease.
Another means of transmission is by ingestion of tissue cysts in raw or undercooked meat (see Fig. 29.1 ). When the cysts rupture within the intestinal tract, bradyzoites are released, enter the intestinal epithelial cells, and differentiate into tachyzoites, which disseminate throughout the body in the intermediate and definitive hosts. To complete the life cycle, bradyzoites can also differentiate into gametocytes in the definitive host cat, leading to development of oocysts, which are shed in feces.
Since the 1980s, it has been recognized that tissue cysts can also be transmitted within a transplanted organ. This scenario occurs more often with heart organs because there is a predilection of T. gondii for muscle. However, tachyzoites can disseminate to all tissues and thus other organs may harbor tissue cysts but at a lower frequency. ,
On a population level, seroprevalence is dependent on the climate and culture of a country. Approximately 20% to 30% of the world’s population is infected with T. gondii. North America has a low seroprevalence (10% to 30%), south and central Europe, including France, has intermediate prevalence (30% to 50%), whereas South America, especially Brazil, has high prevalence (up to 80%). , However, decreasing seroprevalence is being reported in Paris. The incidence of T. gondii disease is dependent on seroprevalence of the population, immunosuppression level, and administration of prophylaxis to an individual. , In transplant recipients, the estimated incidence of T. gondii infection or disease is highest in seropositive allogeneic hematopoietic stem cell transplant (HSCT) recipients, ranging from 2.1% to 19.6%, varying geographically (see Table 29.1 ). This supports reactivation as the strongest risk factor for T. gondii disease in the HSCT population. The incidence is lower for solid organ transplant (SOT) recipients and much lower for oncology and autologous HSCT patients. For the SOT population, donor-recipient mismatch (D + /R − ) is a risk factor for acquiring T. gondii. Within all SOT recipients, D + /R − heart (±lung) transplant recipients have the highest incidence of T. gondii , up to 25% in patients with no prophylaxis. Incidence for noncardiac organ transplant recipients is lower, estimated at 0.08% to 0.2%. There is no risk of transmission with eye, bone, or artery transplants. Although data on vascularized composite allotransplantation are sparse, it would be anticipated that those containing skeletal muscle will be at risk. Mortality rates from the largest study of SOT recipients was shown to be 13%, which is lower than the approximately 38% observed in HCST recipients.
Clinical manifestations
The severity of clinical manifestations of T. gondii disease is dependent on the level of the individual’s immune suppression ( Table 29.2 ). Clinical findings do not distinguish between primary or reactivated T. gondii disease. Fever is common and should prompt the inclusion of T. gondii disease in the differential diagnosis. , Delayed diagnosis is a common theme for fatal cases.
| Clinical Manifestations by Transplant Type | Percent |
|---|---|
| Allo-Hematopoietic Stem Cell Transplant | |
| Disseminated disease | 25 |
| Cerebral toxoplasmosis | 10 |
| Isolated fever | 4 |
| Ocular toxoplasmosis | 4 |
| Solid Organ Transplant | |
| Bone marrow suppression | 63 |
| Central nervous system | 56 |
| Liver | 26 |
| Heart | 23 |
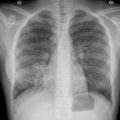
In HSCT recipients, reactivation of persistent tissue cysts is the predominant mechanism leading to clinical disease, manifesting typically in the first 6 months after transplant. In a severely immunosuppressed individual, dissemination of tachyzoites can present as a rapidly progressive infection and can clinically manifest as fever with multiple organ involvement, most commonly the lungs. , For those less immunosuppressed, the brain is the most affected organ clinically and in autopsy. Clinical symptoms of brain involvement include somnolence or obtundation and seizures. Brain abscesses can be detected on imaging. After the brain, lung involvement can be prominent and manifest as a pneumonitis and, in severe cases, evolve to acute respiratory distress syndrome. Computed tomography chest imaging may reveal a reticulonodular or interstitial pattern. The heart and eyes can be affected, manifesting as myocarditis and retinochoriditis, respectively. Cutaneous involvement is rare, presenting as multiple erythematous macules or papules. In some reports, up to 36% of HSCT patients have no symptoms because transient, self-resolving positive T. gondii polymerase chain reaction (PCR) findings have been detected in asymptomatic patients. , ,
Depending on the level of immune suppression, SOT recipients manifest signs and symptoms generally within 3 months after transplant. A broad spectrum of disease can be observed (see Table 29.2 ), including dissemination similar to that seen in severely immunosuppressed HSCT patients as well as isolated organ involvement , , or no symptoms.
The relationship between genotype of T. gondii and clinical findings in immunocompromised patients is controversial. Case reports suggest more severe disease associated with non–type II after allogeneic HSCT, but another study of 88 T. gondii strains showed no differences in clinical outcomes.
Disease prophylaxis and prevention
Pretransplant monitoring
The serologic status of the donor and recipient is helpful in stratifying risk for T. gondii infection and disease. Patients with the highest risk of T. gondii infection and disease are seropositive HSCT recipients and seronegative recipients of a seropositive donor organ (D + /R − ). The benefit of serology screening for organ transplantation may be debatable in low-seroprevalence countries such as the United States, especially with suppressive trimethoprim-sulfamethoxazole (TMP/SMX) therapy. In the United States, serology screening for HSCT and SOT patients varies by institution. In contrast, pretransplant serology screening in France and many European countries is mandatory. Since 2017 all deceased organ donors in the United States are routinely screened for T. gondii, and this additional information may drive the desire to know the recipient’s serostatus, especially with a seropositive donor heart organ. In the United States, routine pretransplant screening for allogeneic HSCT recipients, heart transplant recipients, and selected SOT and oncology patients seems prudent to strategize prevention of T. gondii disease.
Posttransplant monitoring
Serology screening after transplant is not as useful, especially in HSCT recipients. Transient increases of T. gondii –specific immunoglobulin (Ig) G may be due to passive antibody via blood transfusions. In addition, with immunosuppression, the antibody response may be altered or difficult to interpret. In these situations, T. gondii quantitative PCR assays are available and can be helpful. , Routine monitoring of peripheral blood with PCR assays in high-risk situations can detect circulating DNA, sometimes before manifestation of clinical signs and symptoms. A preemptive strategy, with routine serial PCR testing and initiation of treatment if test results are positive, may be useful for a HSCT patient before engraftment when routine suppressive TMP/SMX therapy is not favorable owing to myelosuppressive side effects. In SOT patients, posttransplant serology testing can detect seroconversion with primary T. gondii infection, manifested as a positive T. gondii– specific IgM followed by a positive T. gondii– specific IgG. In a mismatched D + /R − scenario, seroconversion early after transplant suggests graft transmission.
Chemoprophylaxis
TMP/SMX is the most widely used therapy for prophylaxis, although no definitive clinical trial has been conducted to show efficacy ( Table 29.3 ). Despite this, studies have shown that TMP/SMX prevented graft-transmitted T. gondii in D + /R − mismatched scenarios when TMP/SMX was used for Pneumocystis jirovecii pneumonia prophylaxis. , In HSCT recipients, TMP/SMX seems to protect against T. gondii reactivation. In one study, the absence of prophylaxis increased the odds of T. gondii disease and infection approximately 12-fold. TMP/SMX has the added benefit of activity against several other pathogens. Other potential drugs are available for prophylaxis, but their efficacy is not definitively known. In heart transplant recipients, one small study showed that no T. gondii infections occurred after starting pyrimethamine for routine prophylaxis. For HSCT recipients intolerant of TMP/SMX, small studies show protection with weekly pyrimethamine-sulfadoxine and with atovaquone. , None of the current drugs eradicate tissue cysts, but TMP/SMX and atovaquone rapidly kill tachyzoites. Prophylaxis can fail because of impaired absorption or drug interruption from intolerance. Although it is used for prophylaxis against Pneumocystis jirovecii pneumonia, pentamidine has no activity against T. gondii. Prophylaxis should continue for about 6 months in SOT and HSCT patients.
| Medication | Dose Infants/Children | Dose Adults | Formulations | Notes | |
|---|---|---|---|---|---|
| Preferred choice | TMP-sulfamethoxazole | 75 mg/m 2 /dose twice a day 3 times per week on alternate days (2.5 mg/kg/dose twice a day 3 times per week on alternate days) OR 150 mg/m 2 /day once a day for 3 consecutive days per week (5 mg/kg/day once a day for 3 consecutive days per week) OR 150 mg/m 2 /day once a day (dosing based on TMP) | 1 single strength tablet (80 mg) once a day OR 1 double strength tablet (160 mg) once a day 3 times weekly OR 1 double-strength tablet (160 mg) once a day (dosing based on TMP) | Suspension 8 mg/mL (TMP) Tablets Single strength 80 mg (TMP) Double strength 160 mg (TMP) | These doses are also effective as prophylaxis for Pneumocystis jirovecii . If given daily, there is added benefit for preventing infection from bacteria, e.g. Listeria, Nocardia, Salmonella, Haemophilus, Staphylococcus |
| Alternative | Atovaquone | 1-3 months old : 30 mg/kg/day once a day 4-24 months old : 45 mg/kg/day once a day WITH or WITHOUT pyrimethamine 1 mg/kg/day once a day AND leucovorin (folinic acid) 5mg once a day every 3 days >24 months: 30 mg/kg/day once a day Adolescents: 1500 mg once a day Maximum: 1500 mg/day | 1500 mg once a day | Suspension only Mepron 750 mg/5mL (5 mL, 210 mL) Generic 750 mg/5 mL (5 mL, 210 mL) | Administer with food, especially high-fat meal Expensive Mepron contains benzyl alcohol; citrus flavor |
| Alternative | Dapsone (in combination with pyrimethamine and leucovorin) | ≥1 month old: 2 mg/kg/dose once a day (15 mg/m2/day once a day) AND pyrimethamine 1 mg/kg/day once a day AND leucovorin 5 mg once a day every 3 days. | 50 mg once a day (AND pyrimethamine 75 mg once a week AND leucovorin 25 mg once a week) OR 200 mg once a week (AND pyrimethamine 75 mg once a week AND leucovorin 25 mg once a week) | Tablet (scored) 25 mg | Need to test for G6PD deficiency Do not use if severe TMP allergy Use in combination with pyrimethamine because of breakthrough. For HIV-infected patients, dapsone/pyrimethamine is preferred alternative compared to atovaquone based on more data |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree