Although there have been many advances in the treatment of rectal cancer, including improved imaging, chemotherapy agents, and radiation techniques, none has been more important than advances in surgical technique. Among these advances, total mesorectal excision (TME) is probably the most significant.
In order to appreciate the importance of TME, some history of rectal cancer surgery is in order. At the beginning of the 20th century, the vast majority of patients diagnosed with rectal cancer in Europe and the United States underwent perineal proctectomy. While this operation was an improvement over previous surgeries for rectal cancer, it was a morbid procedure with suboptimal oncologic outcomes.
In 1908 the influential surgeon Sir William Ernest Miles, of St. Mark’s Hospital in London, recognized that, following perineal proctectomy, nearly all of his patients died of recurrent disease within 3 years. On autopsy, he noted that most recurrences were found in the part of the mesorectum that had been left in place and/or within lymph nodes situated near the left common iliac artery. Miles termed these areas the “zone of upward spread.”1 He concluded that the woeful inadequacy of perineal proctectomy was due to the fact that it failed to address the ultimate cause of local recurrence: incomplete excision of the mesorectum in its entirety, including its lymphovascular supply.
Based on his observations, Miles devised and began immediately to perform a different procedure, which he described as abdominal perineal excision (APE) or abdominoperineal resection (APR), and this soon became the procedure of choice for surgical treatment of carcinoma of the rectum and the terminal portion of the pelvic colon.1 APR actually involves two procedures performed during the same operation: the abdominal part of the procedure includes dissection of the rectum and mesorectum and creation of a colostomy; the perineal part includes detachment of the rectum and anus from the levators, the genital/urinary organs, and the ischiorectal fat. Compared with perineal proctectomy, mortality and morbidity following this new operation improved dramatically.
Miles’ emphasis on the necessity of removing the mesorectum in its entirety would become the guiding principle of what is now known as total mesorectal excision. Today, TME remains the “gold standard” in the surgical treatment of rectal cancer. As first described by Abel in 1931,2 TME entails sharp—rather than blunt—dissection of the visceral and parietal layers of the endopelvic fascia, resulting in intact removal of the rectum and mesorectum. However, the continued common use of traditional blunt dissection limited the benefits of APR, resulting in a 25% rate of positive resection margins and high rates of recurrence and mortality. The absolute necessity of sharp dissection in every APR—meticulously removing the entire mesorectum along the areolar plane outside of the rectal fascia propria—was reemphasized in 1982 by the surgeon Bill Heald.3 Heald4 defined TME as an “optimal dissection plane around the cancer which must clear all forms of extension and circumscribe predictably uninvolved tissue.” As described by Heald, TME can be basically defined as sharp mesorectal excision along definable tissue planes, with negative margins of resection. Because the rectum and mesorectum constitute a distinct anatomic entity, sharp dissection along the plane between the visceral and parietal layers of the endopelvic fascia achieves mobilization of the rectum en bloc with its intrinsic lymphovascular supply, containing the tumor within the resected mesorectal “package,” or envelope. The aims of TME are to excise the rectum and surrounding mesorectum, including its blood vessels and pararectal lymph nodes, within an intact visceral fascial envelope; to complete en bloc resection of the lymph nodes along the superior rectal and inferior mesenteric arteries (IMAs); and to achieve clear resection margins.
Surgeons, anatomists, and pathologists continued to improve their understanding of rectal cancer spread. In 1985, a seminal paper by Quirke and colleagues showed that relapse was mostly the result of tumor left behind at the circumferential resection margin (CRM). This margin around the mesorectum is one of the most important anatomic elements of rectal cancer surgery. Quirke et al5 were the first to describe a systematic assessment of the CRM in rectal cancer surgery. Demonstrating the strong relationship between CRM involvement by tumor and local pelvic recurrence, they reported that 85% of patients with CRM involvement developed locoregional recurrence versus only 3% of patients with clear margins. This highlighted the importance of achieving a negative margin greater than 1 mm. Since Dr. Quirke’s original publication, numerous studies, including prospective trials, have confirmed that CRM involvement is a powerful predictor not only of local recurrence but also of the development of distant metastases and poor survival.
The quality of a TME resection can be determined by examination of the surgical specimen. If it is intact, the specimen appears bilobal and has a smooth, glistening surface. This indicates a high-quality procedure. A poor-quality specimen will show deep clefts into the mesorectal fat, exposing the muscularis propria. A high-quality TME is associated with much higher rates of survival than a poor-quality (i.e., incomplete) resection.
Total mesorectal excision technique is based on key anatomy of the rectum and the mesorectal fascia. The rectum is located at the end of the large intestine, where the taenia coalesce to form a complete lineal muscular layer; this is surrounded by a recognizable annular envelope called the fascia propria of the rectum—or mesorectal fascia, as it is better known to surgeons. This is also known as the visceral layer of the endopelvic fascia, in distinction to the parietal layer which envelopes the pelvic sidewall. The mesorectum contains the lymphovascular supply of the rectum and upper anal canal. It encloses the branches of the superior rectal artery and the perirectal lymph nodes, which drain in a caudal direction toward the IMA. Around the rectum is an avascular plane, surgically recognizable as a cobweb of areolar tissue.
The perirectal tissue enclosed within the mesorectum is asymmetrically distributed. Most is accumulated on the posterior aspect of the rectal wall and identified by two protruding bulges (the “mesorectal cheeks”), whereas the perirectal tissue anteriorly and laterally is thinner. Similarly, the mesorectal fascia is most developed on the posterior aspect. Anteriorly the mesorectum is thin, and it is bordered by the rectogenital septum known as Denonvilliers’ fascia. In men, Denonvilliers’ fascia separates the rectum and mesorectum anteriorly from the prostate and seminal vesicles. In women, the thinner rectovaginal fascia separates the rectum from the vagina. Ligaments below and lateral to the peritoneal reflection connect to the parietal fascia on the pelvic sidewall. The pelvic autonomic nerves form the superior hypogastric plexus. The hypogastric nerves, arising from splanchnic fibers originating in the T12-L2 spinal junction, are situated near the sacral promontory. The nervi erigentes are situated along the mesorectal fascia posterolaterally. These nerve fibers, originating in the S2-S4 sacral spinal nerve roots, regulate bowel, urinary, and sexual function.6 The inferior hypogastric plexus forms the interlocking fibers of the hypogastric and sacral nerves. This structure, situated on the pelvic sidewall—anterolaterally to the rectum and posterolaterally to the seminal vesicles in men, and in the similar anatomic area in women—appears as a rhombus-shaped plate with numerous openings. Laterally the mesorectum is sometimes incomplete, allowing middle rectal vessels (coming from the internal iliac vessels, present in about 10% to 20% of patients) and autonomic nerves from the inferior hypogastric plexus to enter the rectal wall. Consequently, the mesorectum is tethered inferolaterally to the inferior hypogastric plexus, necessitating a more challenging dissection that is best achieved with precise monopolar diathermy and subtle traction and countertraction, in order to draw the autonomic nerve fibers controlling urinary continence and sexual function carefully away from the surface of the mesorectum, in pursuit of the “holy plane.”
Posterior to the mesorectum is the presacral fascia, which follows the concavity of the sacrum. The presacral fascia is a thickened parietal fascia that covers the presacral veins and fat. It extends laterally to join Denonvillers’ fascia anteriorly. Inferiorly, between the levels of the third and fourth sacral vertebra, the mesorectum and the presacral fascia fuse. The thick connective tissue bridging these two separate fascias is also known as the rectosacral fascia, or Waldeyer’s fascia. It is an important surgical landmark during posterior rectal mobilization, because of its close relationship to the hypogastric nerves and the inferior hypogastric plexus. Inaccurate dissection at this level can lead anteriorly to breach of the mesorectum, and posteriorly to tearing of the fascia, resulting in bleeding from the presacral veins.
Below the levator muscles, at the most distal part of the rectum, the mesorectum thins out as a recognizable structure so that it is virtually absent over the final 1 cm. Tumors at this level may easily breach the plane, invading the external sphincters.
The basic principles of TME are as follows:7
Sharp dissection circumferentially around the mesorectum along an avascular areolar plane between the visceral and parietal layers of the endopelvic fascia (Fig. 110-1).
Identification and preservation of the autonomic nerve plexus that controls bladder and sexual function.
Prevention of tearing of the mesorectum, especially posteriorly when dividing the rectosacral fascia.
Achieving a circumferential margin that is macroscopically clear of tumor (Fig. 110-2). If the tumor extends to the CRM, a more extensive resection is necessary. This would include removal of a portion of the parietal layer of the endopelvic fascia, and any additional anatomic structures involved by tumor.
In the modern era, when sphincter-sparing surgery has become possible for a majority of rectal cancer patients, and APR comparatively rare, the principles of TME also comprise the following:
Extension of rectal dissection deep into the pelvis to achieve an adequate distal margin and preserve the anal sphincter complex.
Restoration of gastrointestinal continuity.
FIGURE 110-1
Sagittal section of male pelvis. The relation of the autonomic nerves to the lateral pelvic sidewall is shown. The hypogastric nerve and the pelvic splachnic nerves join to form the inferior hypogastric plexus. Some nerves leave the plexus and enter the rectal wall, while the remainder continues anteriorly to supply the bladder, prostate, and seminal vesicles. (Reproduced with permission from Gilroy AM: Atlas of Anatomy. 2nd ed. Thieme; 2012.)
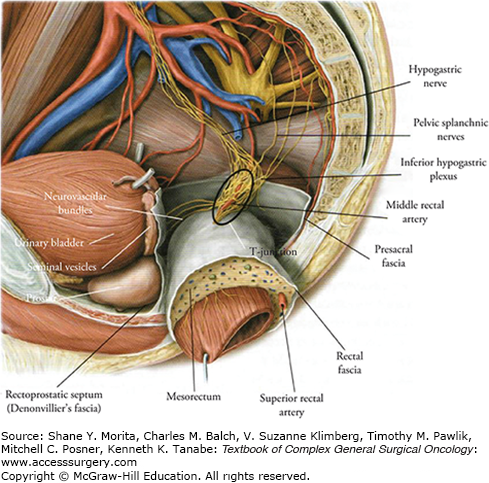
FIGURE 110-2
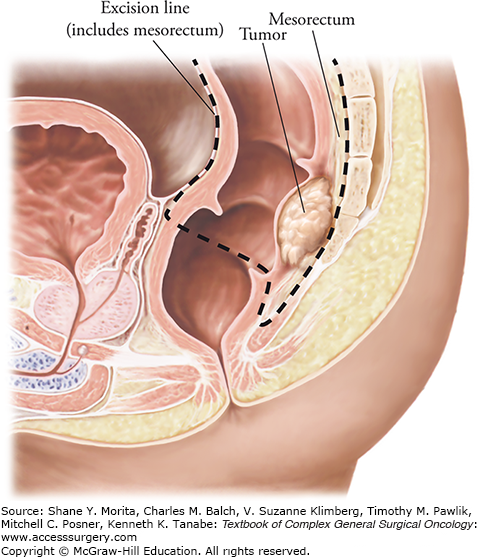
Total mesorectal excision. Dissection follows the dotted line. TME was developed after the recognition that tumor deposits are often present within the lymphovascular tissue that surrounds the rectum (mesorectum). Incomplete resection leaves residual deposits in place and is most likely the origin of local treatment failure. (Adapted with permission from Heald RJ, Moran BJ: Embryology and anatomy of the rectum, Semin Surg Oncol. September 1998;15(2):66-71.)
Although no prospective randomized trial has ever been performed, countless institutional series and cancer registry reports document that adoption of TME reduces local recurrence from double- to single-digit percentages. Furthermore, the type of operation (blunt or incomplete dissection vs. TME) is an independent predictor of local recurrence and overall survival.3,8,9
The quality of TME surgery is demonstrated by the appearance and integrity of the mesorectum in the removed specimen. Quirke and colleagues described a grading system that classifies rectal cancer specimens according to whether the surgeon has dissected outside the mesorectal fascia, in the correct plane (the mesorectal excision plane), or has violated the mesorectum, leaving mesorectal tissue behind in the pelvis by following a plane within the mesorectum (intramesorectal plane) or directly on the muscularis propria (muscularis propria plane). This mesorectal grading system has been evaluated in further studies and has been found to be an independent predictor of local recurrence. One study reported a significant association between plane of surgery and survival—even in patients with an uninvolved CRM.10 However, these studies also showed that the surgical plane was related to CRM positivity rates, with the lowest rates of positive CRM in surgery achieving sharp dissection along the mesorectal plane.
Pathological analysis of the excised TME specimen provides important prognostic information on the stage and biology of the tumor. It is also the ultimate means of assessing the quality of surgery that has been performed.10
The staging of rectal cancer is addressed in depth in Chapter 107, “Locoregional Staging and Restaging of Rectal Cancer.” We will briefly touch upon it here. The preoperative local staging of rectal cancer is primarily done using high-resolution magnetic resonance imaging (MRI) of the rectum. Due to high-quality soft tissue contrast, MRI has been the most useful imaging modality for assessing extent of tumor in relation to the mesorectal fascia, surrounding structures (prostate, seminal vesicles, bladder, uterus, vagina), and anal sphincter complex. MRI has demonstrated reliability in identifying high-risk tumors that involve the mesorectal fascia. In this setting, patients would benefit from preoperative chemoradiation, with the goal of downsizing the tumor so that clear margins may be achieved during TME.11 (Preoperative treatment is discussed in more detail below.) MRI has shown great accuracy in identifying potential tumor at the CRM to within 1 mm.12,13 The excellent accuracy of MRI in delineating the mesorectal fascia—producing results comparable to the results of histological analysis—has also been demonstrated by a large European multicenter trial known as the MERCURY Study.14 In this trial, 349 patients underwent preoperative MRI assessment, followed by TME surgery. MRI was found to be accurate within 0.5 mm, with a specificity of 92% in predicting a clear CRM.
Lymph node involvement can also be assessed with MRI, with accuracy ranging from 60% to 80%. When comparing preoperative MRI findings with surgically resected specimens, Brown and colleagues reported that irregular contour and inhomogeneous signal, rather than size, was the most reliable MRI criteria for predicting lymph node metastasis.11
Preoperative radiotherapy, or chemotherapy with radiation, has become standard practice in the treatment of patients with locally advanced rectal cancer. As mentioned above, high-quality MRI imaging combined with clinical assessment helps multidisciplinary teams identify patients with a “threatened” or involved CRM who will benefit from neoadjuvant treatment.
The two main neoadjuvant strategies currently in use are preoperative short-course radiotherapy (SCRT) and long-course chemoradiation therapy (CRT). SCRT is used mainly in Scandinavia, the Netherlands, and the United Kingdom. It consists of a radiation schedule of 25 Gy delivered in 5 fractions (5 × 5), with surgery performed within a few days of completion of radiation. Delivered over a short period of time, SCRT is of relatively low cost, enabling definitive surgery to take place without delay, and the speedy initiation of systemic chemotherapy (within weeks) after surgery. However, evidence does show that tumor downsizing, in response to radiation, increases over time. Therefore, the potential disadvantage of SCRT is that it may not allow sufficient time for more substantial downsizing and/or downstaging of the tumor before surgery. CRT, on the other hand, consists of long-course preoperative radiotherapy (45 Gy in 25 fractions) with concurrent 5FU-based chemotherapy, followed by surgery 4 to 12 weeks after chemoradiation. The potential advantages of this approach are twofold: it gives the patient more time to recover and may result in greater preoperative tumor shrinkage over time. Downsizing of the tumor increases the opportunity for an R0 resection. CRT is currently used in the United States and in most European countries.
There are some additional theoretical advantages to preoperative compared to postoperative radiotherapy, such as improved radiosensitivity of the tumor cells due to better oxygenation, “sterilization” of the surgical field, leading to reduced seeding and local recurrence, avoiding postoperative radiation of the surgical anastomosis, resulting in less toxicity to the small bowel (which, consequently, is less likely to be involved in the radiation field). To a certain extent these advantages have been confirmed in clinical trials. Neoadjuvant CRT has been evaluated in a German phase III randomized trial that compared preoperative with postoperative CRT in a total of 823 patients. In the German trial, neoadjuvant CRT improved better local control than postoperative CRT (6% vs. 13%, p = 0.006) and was associated with reduced acute and late toxicity; however, it did not improve overall survival.15,16
This being said, there is strong supporting evidence for the superiority of both SCRT and CRT versus surgery alone. The outcomes of SCRT versus surgery alone have been evaluated in randomized trials in the Netherlands and Sweden.17,18 In the Dutch trial, SCRT was found to reduce the rate of local recurrence to 5% versus 11% in the surgery-alone group (p < 0.0001).19 In summary, the rate of local recurrence can be approximately halved with the use of neoadjuvant chemoradiotherapy—whether SCRT or preoperative CRT—when these are combined with TME. Currently, the two strategies appear to be equally effective.
In response to preoperative treatment, some patients achieve a pathologic complete response (i.e., no evidence of viable tumor on pathologic analysis), or pathologic near-complete (>95%) response. Studies have shown that these patients have significantly better overall and recurrence-free survival than those with a lesser response, resulting in improved long-term outcomes. Poor response to preoperative treatment, the presence of lymphovascular and/or perineural invasion, and positive lymph node status are negatively associated with both overall and recurrence-free survival.20,21
The use of preoperative imaging to identify bulky lesions, the use of neoadjuvant therapy, and the adoption of proper TME technique have transformed rectal cancer treatment and improved morbidity and mortality. In the current era, the majority of patients are now candidates for a sphincter-saving procedure, with less than 10% risk of local recurrence, and acceptable maintenance of sexual and bladder function postoperatively. The treatment of bulky, locally advanced rectal cancer is a multidisciplinary undertaking involving surgical oncologists, radiologists, radiation oncologists, medical oncologists, and other subspecialists. Multidisciplinary discussion and consensus regarding the proper plan of treatment is essential in providing each patient with the best chance for optimal outcome.22
Optimal resection of rectal cancer according to the oncological principles of TME can be achieved by open or minimally invasive (laparoscopic or robotic) surgical techniques. Herein we describe our preferred methods for both the open and minimally invasive approaches.
The patient is placed in a modified lithotomy position. A midline incision is made from the symphysis pubis to the mid abdomen above the umbilicus. The abdominal cavity is explored thoroughly, especially the liver and the peritoneum. The small bowel is carefully packed and retracted to the right, providing access to the pelvis. The sigmoid and left colon is mobilized by dissection laterally to medially along the white line of Toldt. The sigmoid colon is retracted medially. In this loose connective tissue plane, first the gonadal vessels and then, more medially, the left ureter are encountered. The dissection is continued in this plane, and the left colon is dissected away from Gerota’s fascia. At the base of the sigmoid mesocolon, the retrorectal avascular plane is entered. While the sigmoid colon is elevated from the left lateral side, gonadal vessels, left ureter, and the left hypogastric nerve are preserved in the embryologic avascular plane, and the mesorectal dissection plane is reached. The sigmoid is retracted in the right lateral direction. Then, from the right side, the sigmoid mesocolon is entered through a window over the surgeon’s hand at the pelvic brim. Through this window, the IMA is liberated, and separate ligations of the artery and vein are performed. The superior rectal artery (just distal to the left colic artery) or IMA at its origin 1 to 2 cm from the aorta is ligated and divided to preserve the sympathetic plexus. High ligation of the IMA is useful when bulky adenopathy is present at the base of the vessel, or when a coloanal anastomosis is necessary and maximal length of the left colon is required. When the IMA is ligated, care must be taken to preserve the marginal artery, which is the blood supply from the middle colic vessels to the left colon and anastomosis. The inferior mesenteric vein (IMV) is ligated at the paraduodenal (ligament of Treitz) location just inferior to the pancreas and again adjacent to the ligation site of the IMA. Dividing the vein at the ligament of Treitz with splenic flexure mobilization is critical to accommodate full mobilization of the descending colon, which is then allowed to rotate into the pelvis for maximal length.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






