
At the time of this chapter release, there were no national guidelines for implementation of specific questions to assess tobacco use in cancer patients. However, Figure 31.1 provides effective questions for assessing tobacco use in cancer patients based on advice from published reports.5,45,194,199 Current, former, and never smokers are identified in a structured manner. Patients who use tobacco within the past 30 days should have structured support to quit tobacco use, maintain abstinence, and prevent relapse. Although not explicitly stated by any specific guidelines, asking about tobacco use in family members of cancer patients may be important because family members often support cancer patients during and following treatment, but continued smoking by family members can make quitting much more difficult.202–204
Advising is the second step in promoting effective tobacco cessation that involves giving clear, strong, and personalized advice to stop tobacco use. This advice should include the importance of quitting smoking, such as explicit information on the risks of continued smoking and the benefits of cessation for cancer treatment outcomes and overall health regardless of cancer diagnosis. This includes a discussion of how it is not “too late” to quit and that quitting will in fact benefit their cancer treatment efficacy and cancer outcome.5 Patients can also consider the cost savings of stopping a smoking habit. Clinicians must be particularly sensitive to avoid contributing to any perceived blame for the patient’s illness.195–197,205 Clinicians must remember that most patients started smoking in adolescence and did not completely understand the risks associated with tobacco use. At the same time, the severe addiction associated with chronic tobacco use makes it difficult to stop.
The next step is assessing dependence and willingness to quit. Asking “How soon after waking do you smoke your first cigarette?” assesses nicotine dependence, with high dependence associated with a shorter interval between waking and the first cigarette.206 Nicotine dependence is predictive of smoking cessation outcomes and can be used as a good indicator of the intensity of cessation treatment needed, such as the need for pharmacotherapy.207,208 Determining the patient’s motivation and interest in quitting are critical parameters that influence the types of intervention strategies to be employed. Different strategies for quitting are based on the transtheoretical model of change and motivational interviewing stance, which recognizes that unique intervention messages and strategies are needed to optimally promote smoking cessation based on a patient’s readiness to quit smoking.209,210 In the general population, recommendations encourage that clinicians set a target quit date within 30 days. However, for cancer patients, the reader is encouraged to consider an urgent need to stop smoking immediately. If patients are unable to quit immediately, then patients should be encouraged to immediately reduce tobacco use and to set a quit date as soon as possible based on the typical need to start cancer treatment in the immediate future.
Assisting patients with smoking cessation involves clinicians helping the patient design and implement a specific quit plan or broadly enhancing the motivation to quit tobacco. Promoting an effective quit strategy for cancer patients should consist of (1) setting a quit date (immediately or as soon as possible), (2) removing all tobacco-related products from the environment (e.g., cigarettes, ashtrays, lighters), (3) requesting support from family and friends, (4) discussing challenges to quitting, and (5) discussing or prescribing pharmacotherapy where appropriate. Patients should also be provided information on cessation support services (Table 31.1). In the cancer setting, patients can also be informed that smoking cessation is a critical component of cancer care over which they have complete control, thereby conferring some personal control over their cancer care.
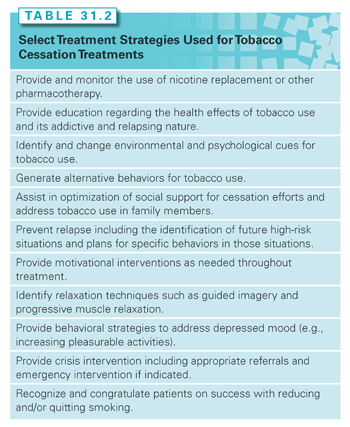
Patients who are unwilling to quit should continue to receive repeated assessments and counseling to help motivate patients to quit smoking. These patients should be encouraged to make immediate reductions in tobacco use and work toward abstinence as soon as possible. Clinician education, reassurance, and gentle encouragement can help them to consider changing their smoking behaviors. Specific strategies include discussing the personal relevance of smoking and benefits to cessation, providing support and acknowledging the difficulty of quitting, educating patients about the positive consequences of quitting smoking, and discussing available pharmacologic methods to assist with quitting.211 The emphasis should be placed on patient autonomy to quit. Motivational strategies for patients unwilling to quit can be employed (e.g., asking open-ended questions, providing affirmations, reflective listening, summarizing).198,210,212,213 Table 31.2 provides suggested methods to help clinicians promote tobacco cessation.
The final step in a clinician-delivered smoking cessation intervention involves arranging a follow-up contact with the patient. Ideally, cancer patients will follow an immediate quit strategy and follow-up should occur preferably within 1 to 2 weeks. However, a short-term follow-up may also benefit patients who are reluctant to quit smoking. The clinician must remember that a new cancer diagnosis is stressful and patients may rely on continued smoking to relieve stress, but after absorbing the psychological effects of a new cancer diagnosis, patients may be more receptive to smoking cessation. During follow-up, clinicians should congratulate patients on successful cessation efforts, discuss accomplishments and setbacks, and assess pharmacotherapy use and problems. Patients should not be criticized for returning to smoking; rather, it is critical to create a supportive environment for patients to communicate progress, failure, and personal needs. Framing relapses as a learning experience can be helpful, and patients should be encouraged to set another quit date. Referrals to a psychologist or professionally trained smoking cessation counselor should be considered for patients with numerous unsuccessful quit attempts, comorbid depression, anxiety, additional substance abuse disorders, or inadequate social support.
Clinicians who are not well versed in tobacco cessation should realize that smoking is an extremely difficult addiction to overcome and should recognize the clinical pattern associated with cessation. As patients stop smoking, many will experience symptoms of withdrawal, including dry or sore throat, constipation, cravings to smoke, irritability, anxiety, trouble concentrating, restlessness, increased appetite, depression, and insomnia. In the first few weeks, patients may also report an increase in mucous secretions from the airways, a cough, and other upper respiratory tract symptoms. Patients and clinicians should realize that tobacco cessation requires a concerted effort, may require repeated attempts, and symptoms will not resolve immediately. Clinicians should counsel patients on a repeated basis, recognize success, and provide repeated assistance if patients relapse.
Pharmacologic Treatment for Smoking Cessation
The principles of pharmacotherapy to help patients quit smoking are fundamentally based on reducing the craving associated with nicotine withdrawal. Nicotine replacement therapy (NRT), in the form of patches, lozenges, inhalers, sprays, and gum, varenicline (Chantix), and bupropion (Zyban) are the three principal first-line pharmacotherapies recommended for use either alone or in combination according to PHS Guidelines.199 Table 31.3 presents information on these first-line agents. Nicotine is the primary addictive substance in tobacco and NRT facilitates smoking cessation by reducing craving and withdrawal that smokers experience during abstinence. NRT also weans smokers off nicotine by providing a lower level and, in some cases, slower infusion of nicotine than smoking.214 Strong evidence from over 100 randomized clinical trials support the use of NRT to increase the odds of quitting approximately twofold as compared with placebo.215 Pooled analyses demonstrate that 17% of smokers receiving NRT were able to quit versus 10% with placebo after at least 6 months. Recent evidence further shows that combination therapy, or dual NRT (such as a nicotine patch and lozenge), is a very effective smoking cessation therapy that produces high quit rates.216,217 Data suggest that activation of the nicotinic acetylcholine receptor (nAChR) may promote tumor development,218 but evidence suggests that the negative aspects of smoking outweigh these concerns.219,220 Furthermore, there are no clinical trials reporting negative outcomes for NRT in cancer patients as related to mortality or recurrence. Studies also demonstrate that NRT is not associated with an increased risk of carcinogenesis in the general population.221,222 As a result, NRT should be used as a clinically proven method to help cancer patients stop smoking.
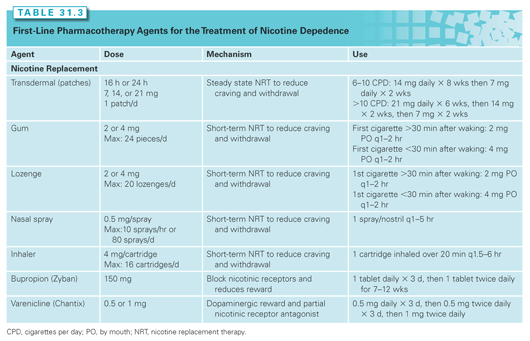
Antidepressants have been studied as non-nicotine–based pharmacotherapy in part due to depression and psychiatric disease being comorbid conditions in smokers.223 Bupropion (Zyban) is currently the only FDA-approved antidepressant for the treatment of tobacco dependence.199 Bupropion inhibits the reuptake of both dopamine and norepinephrine, thereby increasing dopamine and norepinephrine concentrations in the mesolimbic systems.12,224 Bupropion also antagonizes the nAChR, thereby lowering the rewarding effects of nicotine.225 Should an abstinent smoker relapse, bupropion may function to reduce the pleasure of cigarette smoking experienced by the smoker226 and help to prevent further relapse. A meta-analysis found that smokers who received bupropion were twice as likely as those who received placebo to have achieved long-term abstinence at either a 6- or 12-month follow-up.227
Varenicline (Chantix) is a α4β2 nAChR partial agonist that produces sustained dopamine release in the mesolimbic system that received FDA approval for treating tobacco dependence in 2006. Sustained dopamine release maintains a normal systemic level of the neurotransmitter, which helps to reduce craving and withdrawal during abstinence.228 Varenicline also antagonizes the rewarding effects of nicotine. Because varenicline attenuates the pleasure smokers experience from smoking, it may decrease motivation to smoke and protect them from relapse. One of the initially reported randomized clinical trials that compared varenicline (2 mg), bupropion (300 mg), and placebo showed that varenicline was superior to bupropion and placebo, with overall continuous abstinence rates between 10% to 23%.229 A meta-analysis demonstrated that the 1-mg daily dose approximately doubled, whereas the 2-mg daily dose approximately tripled the likelihood of long-term abstinence at 6 months as compared to placebo.199 As a result, the 1-mg daily dose can be considered as an alternative should the patient experience significant dose-related side effects. Several meta-analyses have shown that varenicline is superior to bupropion and placebo in the general population.230–233
In July 2009, the FDA issued a warning after reports that some patients attempting to quit smoking while using varenicline or bupropion experienced unusual changes in behavior, depressed mood, worsening of depression, or had thoughts of suicide. This has prompted recommendations that health-care providers elicit information about a patient’s psychiatric history prior to prescribing varenicline or bupropion to closely monitor changes in mood and behavior during the course of treatment. However, updated recent safety studies examining very large databases (one database of N = 119,546, one database of N = 35,800) regarding safety have shown no difference in neuropsychiatric side effects between varenicline or bupropion as compared to NRT and no increased risk of depression.234,235 Another prospective study showed no adverse events when treating participants with current or past major depression and also showed higher abstinence rates for the varenicline group as compared to placebo at weeks 9 to 52 (20.3% versus 10.4%, p <0.001).236 Varenicline should be considered a viable cessation pharmacotherapy for cancer patients.
The clinical practice guideline also identifies two non-nicotine–based medications—clonidine and nortriptyline—as second-line pharmacotherapies for tobacco dependence. A second-line agent is used when a smoker cannot use first-line medications due to either contraindications or lack of effectiveness. Both clonidine, an antihypertensive, and nortriptyline, a tricyclic antidepressant, have been shown to effectively assist smokers achieve abstinence.227,237 Unfortunately, many patients who quit will eventually relapse, and rates of long-term abstinence remain low. Because smoking poses enormous health risks to individuals and their families, even a modest reduction in smoking may translate into a significant impact on public health. Clinicians should continue to encourage recalcitrant smokers to stop tobacco use and use pharmacotherapy where appropriate with repeated quit attempts.
Empirically Tested Cessation Interventions with Cancer Patients
The overwhelming majority of cessation research has been performed in the general population, but there are several studies that have been performed in cancer patients. Gritz et al.238 conducted the first physician- or dentist-delivered randomized cessation intervention comparison in 186 newly diagnosed head and neck cancer patients. Patients were treated with either minimal advice or an enhanced intervention with trained clinicians consisting of strong personalized advice to stop smoking, a contracted quit date, tailored written materials, and booster advice sessions. No significant differences were found between treatments, but a 70.2% continuous abstinence rate was found at 12-month follow-up regardless of treatment condition, suggesting that many cancer patients can benefit from brief physician-delivered advice. A later study by Schnoll et al.,239 comparing cognitive behavioral treatment with standardized health education advice, also failed to find significant differences in quit rates. All patients received NRT, and quit rates in both groups approached 50% at 1-month follow-up and 40% at 3-month follow-up.
Additional studies, ranging from 15 to 80 patients, examined nurse-delivered cessation interventions for a variety of cancer patients. The lowest cessation rates were found with a single session intervention: a 21% cessation rate in the intervention group versus 14% in the usual care group 6 weeks’ postintervention.240 Higher cessation rates were associated with a more intensive intervention consisting of three inpatient visits, supplementary materials, and five postdischarge follow-up contacts. Additional studies demonstrate higher cessation rates with more intensive intervention (40% to 75%) as compared with usual care (43% to 50%), suggesting more intensive interventions may yield higher cessation rates.241–243 In general, more intense interventions appear to be more efficacious, but even brief advice is important to achieve tobacco cessation.
In a randomized trial of 432 cancer patients coordinated by the Eastern Cooperative Oncology Group (ECOG) with a physician-delivered intervention (comprised of cessation advice, optional NRT, and written materials) or usual care (unstructured advice from physicians), there were no significant intervention effects and generally low abstinence rates (12% to 15% at 6 to 12 months).244 However, patients with head and neck or lung cancer were significantly more likely to have quit smoking compared to patients with tumors that were not smoking related. Analyses of outcomes from the Mayo Clinic Nicotine Dependence Center found that although lung cancer patients were more likely to achieve 6-month tobacco abstinence than controls (22% versus 14%), no significant differences were observed after adjusting for covariates.245 Garces et al.246 also found no significant differences in abstinence rates between head and neck cancer patients and controls (33% versus 26%). However, higher abstinence rates were found for both head and neck and lung cancer patients treated within 3 months of diagnosis compared to those treated for more than 3 months after the diagnosis, emphasizing the potential importance of the teachable moment at the time of the cancer diagnosis.
The potential importance of addressing smoking combined with considering comorbid disease has been noted in a few studies. In a randomized head and neck cancer patients of usual care versus 9 to 11 sessions of a nurse-administered intervention consisting of cognitive-behavioral therapy and medications, targeting comorbid smoking, drinking, and depression significantly increased quit rates at 6-month follow-up for the intervention group compared to the usual control group (47% versus 31%, p <0.05).247 In a randomized trial of 246 cancer patients treated with 9 weeks of NRT with or without bupropion, there was no significant difference with the addition of bupropion to NRT, but in patients with depressive symptoms, bupropion increased abstinence rates, lowered withdrawal, and improved quality of life.248 Patients without depression symptoms did equally well when treated with bupropion versus transdermal nicotine and counseling alone.
Patient recruitment has been a problem noted by some studies, including 5.5 years to accrue 246 patients with telephone screening of over 7,500 potential patients.249 A pilot trial of varenicline in thoracic oncology patients required screening 1,130 patients to accrue 49 participants randomized to a 12-week course of either varenicline or placebo paired with a behavioral counseling platform of seven sessions.250 A randomized trial of 185 smoking cancer patients comparing the efficacy of a hospital-based standard care smoking cessation model versus standard care augmented by a behavioral tapering regimen via a handheld device before inpatient hospitalization for cancer surgery demonstrated no difference in quit rates (both 32%,).251 However, over 29,000 patients were screened to conduct a randomized clinical trial with a smoking cancer patient population. These studies highlight the potential difficulty recruiting participants who smoke, including considerations for the importance of medical comorbidity in guiding smoking cessation treatment, patient mix (multiple tumor sites), treatment status (awaiting treatment to completed treatment), variation in stage of disease, and considering how psychiatric conditions such as depression reflect the difficulty of conducting research in the oncology setting and the importance of these variables in future studies.
Although accruing patients to intervention trials may seem discouraging, several studies demonstrate the benefit of counseling over self-help. Emmons et al.252 conducted a randomized controlled trial in 796 young adult survivors of pediatric cancer that included six calls, tailored and targeted written materials, and optional NRT as compared with self-help. Significantly higher quit rates were found in the counseling group compared to the self-help group at all reported follow-up time points, including 12 months (15% versus 9%; p <0.01). A randomized trial of a motivational interviewing-based smoking cessation intervention in a south Australian hospital was delivered over a 3-month period, consisted of multiple contacts with a trained counselor, and provided supplementary material tailored to cancer patients with NRT.253 The control group received brief advice to quit and generic supplementary material. Quit rates did not differ by treatment group (5% to 6% at 3-month follow-up), but the intervention group was significantly more likely to report attempts to quit smoking.
Current Tobacco Assessment and Cessation Support by Oncologists
Access to cessation support is critical to address tobacco use by cancer patients. A recent survey of 58 NCI-designated cancer centers indicated that about 80% reported a tobacco use program available to their patients and about 60% routinely offered educational materials, but less than 50% had a designated individual who provided services.254 A recent survey of over 1,500 members of the International Association for the Study of Lung Cancer (IASLC)255 and a parallel study of 1,197 ASCO members256 observed that approximately 90% of physicians believe that tobacco affects outcomes, tobacco cessation should be a standard part of cancer care, and approximately 80% regularly advise patients to stop using tobacco, but only approximately 40% discuss medications or assist with quitting. Dominant perceived barriers to cessation support were patient resistance to treatment, an inability to get patients to quit, a lack of cessation resources, and a lack of clinician education. These data showed that even motivated clinicians are not regularly providing tobacco cessation support. A recent survey of 155 actively accruing cooperative group clinical trials further demonstrated that only 29% of active trials collected any tobacco use information, 4.5% collected any tobacco use information at follow-up, and none addressed tobacco cessation.55 Few oncology meetings offer educational workshops or talks, and they are often poorly attended when they are offered.257 Collectively, these data demonstrate that oncologists are not regularly providing cessation support and that we are not capturing tobacco use information that may be critical to understanding the effects of tobacco on cancer treatment outcomes.
More in-person talks as well as written and Web-based training should be made available, as well as new approaches that move from the traditional 5 A’s model delivered by a single professional to referral systems that efficiently connect tobacco users to multiple resources for tobacco cessation.258–260 The ASCO Prevention Curriculum has a chapter devoted to educating oncology health-care professionals on the evaluation and treatment of tobacco use.213 Innovative curricula, such as the Texas Tobacco Outreach Education Program (TOEP), are available and can facilitate program development in other states.261 However, specialty programs in tobacco cessation treatment that are based in cancer centers and other medical centers are valuable resources that need to be further developed.
Addressing tobacco use in cancer patients may be approached in a systematic and efficient manner. A recent report highlighted the potential utility of automated tobacco assessment and smoking cessation using structured assessments in the EMR where all patients were automatically referred to a dedicated cessation program consisting of phone-based cessation support.45 In 2,700 patients referred for cessation support, half received only a mailing and only 1% contacted the cessation program. However, in the arm with at least five phone call attempts made by the cessation service, 81% of patients were successfully contacted and only 3% refused cessation support. Furthermore, assessments implemented every 4 weeks, rather than more frequent assessments every 2 weeks, resulted in delayed cessation referrals in less than 1% of smokers. This is the first report to try and identify clinically efficient mechanisms of addressing tobacco use that may be useful in clinical practice or research that may be an effective method of increasing patient participation in cessation support, but substantial work is needed to assess who may benefit from low versus high intensity support in such a program.
Examples of Model Tobacco Treatment Programs
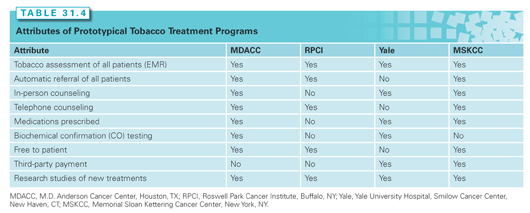
Several dedicated tobacco treatment programs at cancer centers have been developed. Table 31.4 contrasts the core elements of four active model programs at the end of 2013 (University of Texas M.D. Anderson Cancer Center, Roswell Park Cancer Institute, Yale Cancer Center, and Memorial Sloan Kettering Cancer Center), each of which employ different methods to help cancer patients quit smoking. All programs follow the evidence-based 5 A’s model described previously from PHS Guidelines.199 All programs were made available to patients at their respective medical centers and are now designed to evaluate and treat all patients who self-report current tobacco use. Importantly, not all cancer centers can treat smoking cessation in the same manner. Financing of a cessation program is critical and may include institutional funds, state funds, research funds, and third-party billing. Notably, given the broad spectrum of adverse health effects associated with smoking, cancer centers should carefully consider the potential health benefits and cost savings associated with tobacco cessation due to reductions in treatment complications and recurrence associated with smoking by cancer patients. There is no one “correct” way to create and sustain a tobacco treatment program at a cancer center, but at the very least and consistent with evidence, rigorous behavioral counseling should be provided and, if possible, medication management as well.
Research Considerations
The past several years have shown a surge in activities identifying the effects of tobacco in cancer patients and increasing awareness is being developed for cessation support at cancer centers as well as through several national organizations. There are three fundamental areas of research that need to be expanded:
1. Evaluating the effects of tobacco use and cessation on clinical cancer outcomes. The 2014 SGR concluded that smoking caused adverse outcomes in cancer patients,7 but several limitations remain. Tobacco-use definitions should be standardized and implemented at diagnosis, during treatment, and follow-up. Biochemical confirmation with cotinine or exhaled carbon monoxide may improve the accuracy of tobacco assessment in at-risk groups such as current smokers who are trying to quit or patients who reported quitting in the past year.171,185,186,262 Although smoking is the predominant form of tobacco consumption, all tobacco products should be considered. A further understanding of the effects of tobacco on the efficacy and toxicity of cancer treatment, tumor response, quality of life, survival, recurrence, compliance, second primary, and noncancer-related comorbidity is needed. All cancer disease sites and stages are important to consider.
2. Understanding the effects of tobacco and cessation on cancer biology. Although not a primary focus of this chapter, tobacco and tobacco-related products increase tumor growth, angiogenesis, migration, invasion and metastasis and decrease response to conventional cancer treatments such as CT and RT. These and other areas are important to consider, including the potential effects on immune-related therapy and vaccine development. In vivo models of exposure and cancer response are not well developed, yet are critical to this research area. Work is also needed to assess the effect of emerging tobacco-related products such as e-cigarettes.
3. Advance understanding of models to increase access to cessation support and increase efficacy of tobacco cessation methods for cancer patients. This diverse area includes assessing the timing of intervention, intensity, duration, follow-up, and the potential effects of harm-reduction strategies. Cessation pharmacology requires additional consideration in combination with unique approaches to motivational and behavioral counseling in cancer patients. Significant work is needed to disseminate evidence-based cessation support and to assess the cost-effectiveness of different cessation strategies, particularly with regard to improving the cost of cancer care as a whole. Preventing relapse and evaluating the safety of transition to alternative products such as e-cigarettes is equally important and increasingly complex with the addition of new tobacco-related products. Identifying and addressing barriers to effective cessation support is also needed. As related to the cancer patient, clinicians and cessation specialists should consider how their research relates to cancer care. Taking advantage of new integrated medical management systems presents a significant opportunity to improve cessation support access as well as to develop a more effective tracking of patient outcomes.
Policy Implications and Systematic Issues
Several national and international organizations have emphasized the importance of tobacco assessments and cessation for the general population and for cancer patients that include tools to evaluate tobacco use at diagnosis, during treatment, and follow-up appointments, as well as routine support for smoking cessation.5,6,188–191 In 2012, ASCO, with the contribution of the American Legacy Foundation, published a Tobacco Cessation Toolkit for the oncology setting.263 This evidence-based guideline intends to help oncology providers integrate tobacco cessation strategies into their patient care. Utilization of the EMR and standardized, automated systems for more efficacious and efficient access to tobacco cessation support has also been suggested,45 but requires participation by clinicians, institutions, insurers, and health departments. Not only should providers be aware of the need for tobacco cessation and available interventions, but health-care institutions must also build such treatment into their overall system of care. Thus, the identification of patients who smoke or use any alternative tobacco product, referral or direct treatment by providers, billing and reimbursement for treatment provided, and consistent efforts from professional oncology organizations are critically important.257 The tremendous public health burden from tobacco-related disability and death has not been countered by a proportional level of funding in tobacco control, cancer treatment research, or public advocacy. Researchers, clinicians, and advocates must come together to persuade policy makers to increase funding in tobacco-related research, treatment, and policy initiatives on behalf of healthy individuals and patients. A united front is critically needed in support of a common agenda that includes both increased tobacco-control efforts and additional funding for disease-related research and treatment. With clinical rationale, guidelines, and advocacy in place, the final steps in effective tobacco control and improving health outcomes are to implement these recommendations into practice.
R E F E R E N C E S
1. U.S. Department of Health, Education, and Welfare. Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. PHS Publication No. 1103. Washington, D.C.: U.S. Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control; 1964.
2. Office of the Surgeon General, Office on Smoking and Health. The Health Consequences of Smoking. A Report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention; 2004.
3. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention; 2010.
4. U.S. Department of Health and Human Services. The Health Consequences of Smoking: Nicotine Addiction. A Report of the Surgeon General
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








