Thyroid Diseases: Introduction
Thyroid disorders in the elderly population are common, often challenging diagnostically, and frequently overlooked. The clinical presentations of thyroid diseases maybe subtle, with nonspecific signs and symptoms that are attributed to other illnesses or to a normal aging process. Thyroid function tests can be misleading in the presence of concurrent acute or chronic diseases and may be affected by some medications. This chapter describes the most common thyroid disorders encountered in the elderly population.
The Aging Human Thyroid
The thyroid gland is the largest endocrine organ in the human body, and weighs approximately 12 to 20 g in adults. The structural and functional changes of the thyroid gland that occur with aging are controversial. Some investigators report that there were no size or weight changes, others found increases to twice normal size after age 70 years, whereas other reports indicated that the thyroid gland undergoes atrophy, fibrosis, and decrease in weight. The thyroid gland is also more nodular with advancing age, and there is an increase in fibrosis and lymphocytic infiltration. Despite these changes, normal thyroid function is maintained by the vast majority of the elderly population. Estimation of the thyroid size and its palpation may be difficult in elderly patients because of cervical kyphosis, obesity, or chronic pulmonary disease.
Iodine, an essential substrate for synthesis of thyroid hormone, is absorbed from the diet and enters the circulation as inorganic iodide that is distributed in extracellular fluids as well as in salivary, breast, and gastric secretions. The average daily iodine intake is about 250 μg/day in the United States. A 24-hour urinary iodine measurement is an index of dietary iodine intake.
Iodide is actively concentrated by the thyroid gland or cleared from the plasma by the kidney. The thyroid gland, compared with the kidneys, is the active participant in the competition for plasma iodide and adjusts the rate of entry of iodide into the thyroid tissue based on the changes in thyroid hormone synthesis rather than renal avidity for iodide ion. The active transport of iodide from plasma to follicular cell is carried out by the sodium iodide symporter, a transport protein on the follicular cell plasma membrane. The extracellular fluid iodide concentration is usually very low because of the rapid clearance of iodide from extracellular fluid by the thyroidal uptake and renal clearance.
Table 108-1 summarizes the aging effects on thyroid function. The renal and thyroidal iodide clearance rate diminishes with advancing age. Thyroid iodide clearance, estimated by a 24-hour radioactive iodine uptake by the thyroid gland, decreases in euthyroid subjects after age 60 years. Urinary iodine excretion also was found to be significantly reduced in subjects older than 80 years of age.
Renal iodide clearance ↓ |
Thyroid iodide clearance ↓ |
Total T4 production ↓ |
T4 degradation ↓ |
Serum T4 concentration ↔ |
Serum TBG concentration ↔ |
T3 concentration ↓ |
Reverse T3 concentration ↑ |
TSH response to TRH ↑↔↓ |
Diurnal variation of TSH ↓ ↓ |
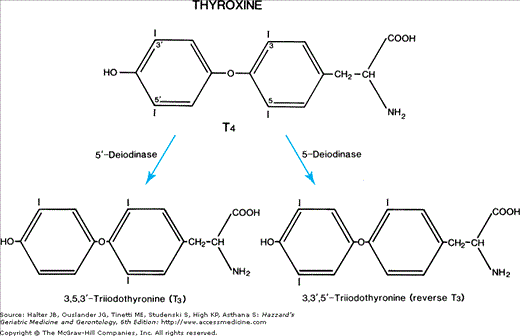
In the thyroid cell, the iodide is oxidized by the peroxidase enzyme and incorporated into tyrosines in thyroglobulin to form the thyroid hormone precursors monoiodotyrosine (MIT) and diiodotyrosine (DIT). The MIT and DIT within the large thyroglobulin molecule couple to form thyroxine (T4) and triiodothyronine (T3). In the plasma, the main binding protein is thyroid-binding globulin (TBG), which binds about 70% of serum T4 and T3. The other binding proteins are transthyretin and albumin. Only 0.02% of T4 and 0.3% of T3 are free and metabolically active because only the free hormone is rapidly transported into cells. Total serum T4 and T3 are readily measured. Free T4 (FT4) and free T3 (FT3) concentrations also can be measured to evaluate thyroid function.
The daily production rate of T4 is about 85 μg/day and that of T3 is about 30 μg/day in normal adults. About 85% of T3 production is derived from T4-to-T3 conversion by 5′-deiodinase, a selenoprotein (Figure 108-1), in extrathyroidal tissues such as the liver, muscles, and kidneys. The other 15% of T3 production is that secreted directly by the thyroid gland. Selenium deficiency results in reduced 5-deiodinase activity and serum T3 concentration. Reverse T3 (rT3), inactive biologically, differs from T3 because it is missing an iodine from the inner or tyrosyl ring of T4 rather than from the outer ring or phenolic ring. T4 is converted to rT3 by 5-deiodinase in peripheral tissue. Total T4 production and degradation decline with aging, but T4 concentration and TBG concentration remain unchanged in healthy individuals throughout adult life. In contrast, the concentration of T3 was reported to decrease by 10% to 20% with advancing age and the concentration of rT3 increases. These findings suggest that 5′-deiodinase activity decreases with increasing age.
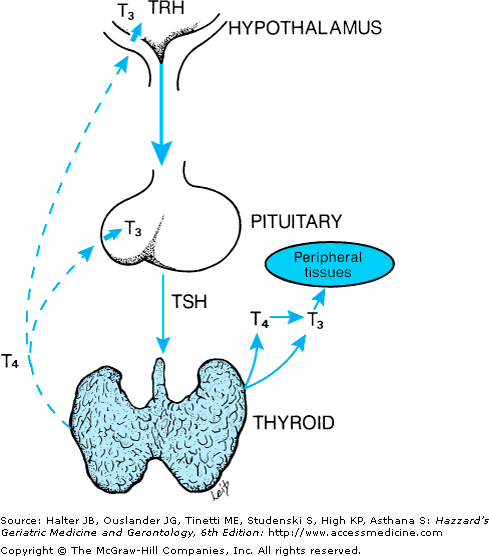
Thyroid hormone regulation is through a negative feedback loop involving the hypothalamus, the anterior pituitary, and the thyroid gland (Figure 108-2). Thyrotropin-releasing hormone (TRH), synthesized and stored within the hypothalamus, stimulates the release of thyroid-stimulating hormone (TSH) from the anterior pituitary gland. TSH binds to the TSH receptor located on the outer side of the thyroid cell plasma membrane and increases thyroid hormone synthesis and secretion. In turn, T4 and T3 from the serum feedback on the pituitary and the hypothalamus to inhibit TSH and TRH production and secretion.
Figure 108-2.
Feedback regulation for control of thyroid function that involves the hypothalamus–pituitary–thyroid axis. Arrows represent positive feedback; dashed lines denote the inhibitory feedback of T4 and T3 on pituitary thyroid-stimulating hormone (TSH) and hypothalamic thyrotropin-releasing hormone (TRH) secretion.
The secretory response of TSH to TRH stimulation in aging men has been reported to be decreased to 38% of the values in young men. This maybe an adaptive mechanism to the reduced need for thyroid hormone in old age. However, other reports of TRH-stimulated TSH secretion with aging have shown an unchanged or even increased response.
The serum TSH concentration has been either unchanged, lowered, or increased with aging in various reports. The heterogeneity of the populations studied may explain some of these discrepancies. Studies employing sensitive TSH assays have raised the question of whether the abnormal TSH reflects the prevalence of thyroid disorders or physiologic changes related to aging. In a random selection of the community-based population followed in the Framingham Heart Study, euthyroid older persons were found to have the same level of TSH as younger persons, although older euthyroid women had a slightly lower serum TSH level than middle-aged women. In a study of healthy centenarians (age range, 100–110 years), the median serum TSH level was lower than that of older individuals (age range, 65–80 years). The data of this study are consistent with TSH being well preserved until the eighth decade of life in healthy elderly subjects, whereas a decline in TSH may occur in those older than 100 years of age. TSH levels rise about 50% in the late evening before the onset of sleep. Sleep attenuates this nocturnal peak of TSH secretion, and sleep deprivation exaggerates nocturnal TSH secretion. The diurnal variation of TSH levels has been reported to be absent in the elderly. The data from the healthy centenarians and individuals older than age 65 years also showed an age-related blunting of the nocturnal TSH peak. An increased prevalence of thyroid autoantibodies is also associated with human aging.
Screening for Thyroid Disease
Both functional and anatomic abnormalities of the thyroid gland occur with increasing prevalence as patients age, and may present with nonspecific clinical findings. Therefore, the clinician should maintain a low threshold for testing if a patient presents with symptoms or signs that suggest the presence of thyroid disease or with atypical clinical findings (e.g., unexplained weight loss caused by apathetic hyperthyroidism). Testing should also be carried out in patients with a prior history of thyroid disease, other autoimmune disease, unexplained depression, cognitive dysfunction, or hypercholesterolemia.
Whether truly asymptomatic older subjects should be screened for thyroid disease is more controversial. The American Thyroid Association recommends screening all adults older than age 35 years for thyroid dysfunction and every 5 years thereafter. However, the American College of Physicians does not recommend routine screening of asymptomatic patients because of presumed lack of demonstrated efficacy or proven benefit in treatment of subclinical thyroid disease. In our opinion, the high prevalence of hypothyroidism and often subtle or nonspecific symptoms in patients older than 65 years of age justifies periodic screening for hypothyroidism.
In most ambulatory patients, the measurement of a serum TSH level is sufficient to screen for thyroid dysfunction. Modern TSH assays are sufficiently sensitive to distinguish normal from low or high values. TSH levels become abnormal before serum T4 or T3 levels because of the exquisite sensitivity of the pituitary gland to small increments in thyroid hormone feedback. However, there are certain patient populations where TSH levels alone may not provide accurate information about thyroid function. Patients with pituitary or hypothalamic disorders may have altered thyroid function with misleading TSH levels, and a full panel of thyroid tests is required to characterize their thyroid function. More commonly, patients with serious acute or chronic illnesses or receiving certain drugs may have altered thyroid hormone and TSH levels that do not accurately reflect their thyroid function. These common scenarios are described in the sections below on nonthyroidal illness and drug effects on thyroid function.
Nonthyroidal Illness
The terms sick euthyroid syndrome or nonthyroidal illness (NTI) refer to altered serum thyroid hormone concentrations secondary to the physiologic stress of severe illness. By definition, patients with NTI have no apparent intrinsic thyroid disease. The types of illnesses responsible for thyroid function abnormalities include sepsis, surgery, trauma, burns, infections, malignancy, and chronic metabolic diseases such as malnutrition, starvation, and poorly controlled diabetes mellitus. An understanding of the effect of NTI on thyroid function tests is important, especially in the elderly patient who has multiple other underlying medical problems.
The effects of NTI on thyroid function have been described as the low T3 and low T4 states. The low T3 state is associated with a decrease in extrathyroidal T3 production, resulting in a low serum total T3 level and usually low free T3 level with a normal serum TSH concentration. With more severe illness, the serum T4 level decreases. In severe NTI, the decreases in T4 and T3 maybe an adaptation to spare the patient from the catabolic effect of thyroid hormone during the periods of extreme stress. A reduction in serum T3 concentration is the most common change of thyroid function tests in NTI with a frequency of 25% to 50%. The severity of the underlying illness correlates with the degree of the fall in serum T3 concentration. The mechanisms responsible for low T3 concentration are (1) a decrease in the peripheral conversion of T4 to T3 either because of inhibition of the 5′-deiodinase that is responsible for this conversion or because of a deficiency of a cofactor, such as glutathione, which is necessary for the activity of 5′-deiodinase; (2) a decrease in T3 secretion from the thyroid gland; and (3) a decrease in tissue uptake of T4 that limits the conversion of T4 to T3 in the extrathyroidal tissues. The serum rT3 is increased in NTI because of the impaired rT3 clearance as a consequence of the decreased activity of 5′-deiodinase with illness. The central question is how does the body maintain a euthyroid state when serum T3 is reduced? The basis for an apparent euthyroid status in NTI is still unclear. There are several possible explanations: (1) T3 concentrations may remain normal in the intracellular compartment even though serum T3 level is decreased; (2) T3 maybe converted to triiodothyroacetic acid (Triac), which is metabolically active; and (3) studies of patients with various acute illnesses showed an increase in T3-receptor messenger ribonucleic acids (mRNAs) and T3-receptor protein that results in an increase in production of proteins that express the action of thyroid hormone, hence maintaining a euthyroid state in NTI.
The patients with the low T4 state, also known as a low T3/T4 state, exhibit a low serum thyroxine level as well as low T3 with a normal serum TSH concentration. Low serum total T4 correlates with a poor prognosis. The mortality of critically ill patients with NTI is inversely related to serum T4 concentration and has been reported to be as high as 84% in patients with serum T4 concentration less than 3 μg/dL. There is no clear mechanism to fully explain the low T4 state; however, possibilities include (1) reduced TBG concentration as a consequence of reduced hepatic protein synthesis; (2) inhibition of serum T4 binding to TBG, probably by a substance released by injured tissue, or an acquired structural alteration of TBG that reduces its affinity for T4; (3) alterations in hepatic uptake and metabolism of T4; and (4) reduced secretion of T4 caused by alteration in the structure of TSH, resulting in decreased biologic activity. Proinflammatory cytokines produced by the mononuclear cells (macrophages, lymphocytes, and monocytes) of the immune system in patients with NTI are probably responsible for the changes in thyroid function tests. Administration of proinflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1 to experimental animals made the animals sick and reduced serum T4, T3, and TSH concentrations. These cytokines inhibit thyroid iodide uptake by reducing the activity and transcription of the sodium iodide symporter, and they inhibit T3 production by reducing the activity and transcription of 5′-deiodinase.
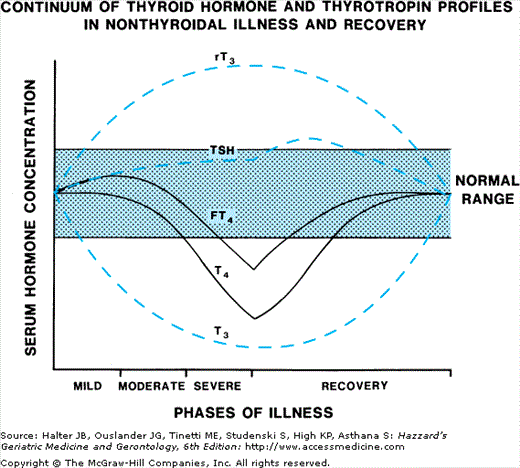
Recovery from the underlying illness results in improvement of the low T3 and T4 states. Serum T4 level returns to normal faster than serum T3 level. Serum TSH concentration usually remains normal except in those patients receiving pharmacologic doses of dopamine or glucocorticoids, which reduce serum TSH levels. During the recovery stage, serum TSH usually remains in the normal range, but it may transiently increase above the normal range. Figure 108-3. diagrams the changes in thyroid hormone and serum TSH levels in NTI.
Figure 108-3.
The relative changes in serum thyrotropin and thyroid hormone concentrations with increasing severity of nonthyroidal illness and with recovery. Serum T4 and free T4 falls with more severe illness, whereas serum T3 is subnormal in mild illness. The recovery is generally a reverse of the illness pattern with a slight elevation of serum TSH in many instances.
The effects of thyroid hormone replacement in NTI have been studied. Treatment of the low T3 state with replacement doses of T3 was found to be detrimental in a fasting model of NTI, resulting in an increase in protein catabolism and possibly muscle breakdown. T3 given intravenously to cardiac patients undergoing open-heart surgery improved cardiac performance, but this was not confirmed in randomized trials. T4 therapy in severe NTI had no beneficial effects and did not improve survival.
Drugs and Thyroid Function
Altered sensitivity to drugs is particularly relevant in elderly patients with thyroid diseases. The metabolism and secretion of many drugs are attenuated in hypothyroidism and accelerated in thyrotoxicosis. Hypothyroidism results in increases in the plasma half-life of digoxin, insulin, glucocorticoids and morphine; consequently, sensitivity to the toxic effects of these drugs increases and doses should be decreased until the patient is euthyroid. Opposite metabolic changes in hyperthyroidism result in increased maintenance doses of these drugs while the patient is hyperthyroid. Resistance to the anticoagulant effect of warfarin in hypothyroidism is a result of slower-than-normal clearance of vitamin K-dependent coagulation factors; an augmented response is seen in hyperthyroidism.
Medications can affect many aspects of thyroid hormone secretion, absorption, transport, or metabolism, as listed in Table 108-2. A few drugs have been shown to suppress TSH secretion, most commonly seen in patients with critical illnesses receiving glucocorticoids and/or dopamine who can have low or undetectable TSH levels as a result. Low TSH levels are also seen during octreotide therapy for acromegaly or other rare endocrine diseases, and during administration of the retinoid X receptor ligand bexarotene for malignancies. Some of these patients develop central hypothyroidism and require thyroxine therapy.
Decrease TSH secretion |
Dopamine |
Glucocorticoids |
Octreotide |
Bexarotene |
Increase thyroid hormone secretion |
Iodine and iodine-containing compounds |
Amiodarone |
Lithium |
Interferon alpha and IL-2 |
Decrease thyroid hormone secretion |
Thionamides (proplythiouracil, methimazole) |
Lithium |
Iodine and iodine containing compounds |
Amiodarone |
Aminoglutethimide |
Interferon alpha and interleukin 2 |
Sunitinib |
Decrease T4 absorption |
Calcium |
Proton pump inhibitors |
Cholestyramine, Colestipol |
Aluminum hydroxide, sevelamer |
Ferrous sulfate |
Sucralfate |
Raloxifene (?) |
Increase serum TBG |
Estrogen |
Tamoxifen and raloxifene |
Clofibrate |
Fluorouracil and capecitabine |
Mitotane |
Heroin |
Methadone |
Decrease serum TBG |
Androgen |
Anabolic steroids (danazol) |
Glucocorticoid |
Inhibit thyroid hormone binding to transport proteins |
Phenytoin and carbamazepine |
Furosemide |
Salicylates and salsalate |
Fenclofenac and meclofenamate |
Heparin |
Sulfonylureas |
Decrease T4 5′-deiodinase activity |
Propylthiouracil |
Amiodarone |
Glucocorticoids |
Increase hepatic T4 and T3 metabolism |
Phenobarbital |
Rifampin |
Phenytoin |
Carbamazepine |
Sertraline |
A second major category of drugs that affect thyroid function include agents that directly increase thyroid hormone secretion. This may occur as a result of stimulation of a thyroid gland with underlying autonomous function, such as latent Graves’ disease or a multinodular goiter. Iodine, iodine-containing radiocontrast agents, and amiodarone all act in this way to increase thyroid hormone synthesis and precipitate hyperthyroidism. A second mechanism is induction of destructive thyroiditis, leading to release of preformed thyroid hormone and transient thyrotoxicosis, as can occur with admiodarone, lithium, or the cytokines interferon alpha and IL-2.
Another class of drugs decreases thyroid hormone secretion. The thionamides proplythiouracil and methimazole are potent inhibitors of thyroid hormone synthesis and are used to treat hyperthyroidism. This class also includes iodine and iodine-containing agents, which can suppress thyroid hormone secretion in patients with underlying defective thyroid glands (e.g., Hashimoto’s disease). It also includes some of the same drugs that cause thyroiditis, including amiodarone, lithium, and interferon alpha and IL-2. During drug-induced thyroiditis, the initial thyrotoxic phase is followed by a phase of reduced thyroid hormone secretion until the injured gland can recover synthetic function. The tyrosine kinase inhibitor, sunitinib, used to treat renal cancer and gastrointestinal stromal tumor, and aminoglutethamide, used to treat adrenal cancer, have also been reported to decrease thyroid hormone secretion.
A number of agents decrease thyroid hormone absorption from the gastrointestinal tract, including drugs commonly prescribed for elderly patients like calcium, ferrous sulfate, and proton pump inhibitors. These drugs do not affect thyroid hormone levels in euthyroid subjects, but they can lead to thyroxine malabsorption in patients taking exogenous thyroxine. Thyroxine should be given separately from these drugs, and the dose may have to be increased as well.
Drugs that cause increased serum TBG levels lead to increases in serum total T4 and total T3. Free T4, free T3, and TSH levels remain normal, attesting to the patient’s euthyroid state. The most common cause of increased serum TBG and T4 concentrations in postmenopausal women is estrogen replacement therapy. The serum TBG concentration is increased by 30% to 50% in women receiving 0.625 mg of conjugated estrogen daily. Less common are drugs that lower serum TBG (and therefore total T4 and total T3) levels.
Other drugs inhibit thyroid hormone binding to TBG and other transport proteins. Like agents that decrease TBG levels, this inhibition causes low total T4 levels, with normal free T4 and TSH levels, with the patient remaining euthyroid. The most frequently prescribed drugs in this category include high-dose aspirin or salsalate, high-dose furosemide, and heparin.
Drugs that inhibit 5′ deiodinase activity include propylthiouracil, amiodarone, propronolol, and glucocorticoids. These agents block T4 to T3 conversion, but do not lead to clinical thyroid disease in euthyroid subjects.
The activity of the hepatic microsomal enzymes that metabolize T4 and T3 is increased by phenobarbital, rifampin, phenytoin, and carbamazepine. Hypothyroid patients treated with levothyroxine may become hypothyroid again when these agents are administered, and some patients require substantial increases of thyroxine dose.
Hypothyroidism
Hypothyroidism is a general term that refers to a state of decreased thyroid hormone availability to peripheral tissues. Overt hypothyroidism occurs when serum FT4 levels are below the normal range and usually is associated with some symptoms of hypothyroidism. Mild thyroid failure with an elevated serum TSH level and normal FT4 concentration is referred to as subclinical (biochemical) hypothyroidism. Subclinical hypothyroidism is discussed in depth at the end of this section.
The prevalence of hypothyroidism varies based on the population under study (i.e., geriatric inpatient vs. primary care setting), age range, ethnicity, iodine content of the diet, and prevalence of antithyroid antibodies. In patients older than 60 years of age in the general population, the incidence of overt hypothyroidism is 2.3% to 10.3%. As dietary iodine intake increases, these rates also increase as a result of iodine effects to suppress the thyroid gland. In a comparative study of healthy elderly female patients, the prevalence of hypothyroidism in Regio Emilia, Italy, where there is low dietary iodine intake, was 0.9%, whereas a prevalence of 14% was found in Worcester, Massachusetts, where the dietary iodine is much higher.
Table 108-3 lists the main causes of hypothyroidism. Primary hypothyroidism accounts for the vast majority of cases of thyroid failure. Less than 1% of cases are caused by central hypothyroidism.
Primary hypothyroidism |
Chronic autoimmune thyroiditis (Hashimoto’s thyroiditis) |
Radiation |