Malnutrition
Protein-energy malnutrition (PEM), the primary focus of this chapter, is present when insufficient energy and/or protein is available to meet metabolic demands. PEM may develop because of poor dietary protein or calorie intake, increased metabolic demands as a result of illness or trauma, or increased nutrient losses.
Maintenance of nutrition is an essential component of comprehensive geriatric care, particularly in the acute care setting where the presence of malnutrition is clearly associated with increased complications and other adverse health outcomes. Prevalence data, relying on a variety of measures of nutritional adequacy, suggest that deficiencies in macronutrients (protein-energy intake) and micronutrients (vitamins and minerals) are very common among older adults. National survey data indicate that 40% to 50% of noninstitutionalized older adults are at moderate to high risk for nutritional problems, and that up to 40% have diets deficient in three or more nutrients. Prevalence estimates in selected populations over 65 years old indicate that 9% to 15% of older persons seen in outpatient clinics, 12% to 50% of hospitalized elderly persons, and 25% to 60% of older persons residing in institutional settings have one or more nutritional inadequacies—with PEM being the most common. Physical and psychosocial factors that may lead to inadequate nutrition are listed in Table 40-1.
Socioeconomic | Physiologic |
Fixed income | Impaired strength/aerobic capacity |
Reduced access to food | Impaired mobility/dexterity (arthritis, stroke) |
Social isolation | Impaired sensory input (smell, taste, sight) |
Inadequate storage facilities | Poor dentition/oral health |
Inadequate cooking facilities | Malabsorption |
Poor knowledge of nutrition | Chronic illness (via anorexia, altered metabolism) |
Dependence on others | Alcohol |
Caretakers Institutions | Drugs (e.g., SSRIs,* NSAIDs,† digoxin, opiates, levodopa, antibiotics, metformin, iron, others) |
Psychological | Acute Illness/Hospitalization |
Depression | Failure to monitor dietary intake and record weights |
Bereavement | Failure to consider increased metabolic requirements |
Anxiety, fear, paranoia | Iatrogenic starvation (e.g., NPO‡ for diagnostic tests) |
Dementia | Delay in instituting nutritional support |
Energy intake does decline significantly with age, attributable to the decrements in lean body mass and physical activity that often accompany aging. A still greater reduction in caloric intake to levels that may be below daily requirements has been a consistent finding of nutritional surveys conducted among community-dwelling elderly people. In the most recent National Health and Nutrition Examination Survey (NHANES III), the mean daily energy intake of persons aged 70 years and older was approximately 1800 kcal/d for men and 1400/d kcal for women, and more than 10% of elderly people reported consuming less than 1000 kcal/d. Even if this limited energy intake met the caloric needs of less-active older adults, it is unlikely that all noncaloric nutrient needs (vitamins and minerals) would be met unless the diet was extremely diverse and rich in nutrients. Although micronutrient deficiencies are common when PEM is moderate to severe, it is the PEM that tends to have the greater clinical impact. Accordingly, this section focuses primarily on PEM, with particular attention to prevention and management of PEM in the acute care setting.
Poor nutritional status and PEM are associated with altered immunity, impaired wound healing, reduced functional status, increased health care use, and increased mortality. Despite the confounding effect of coexisting nonnutritional factors, many studies indicate that poor nutrition remains an independent source of increased morbidity and mortality after adjustment for nonnutritional factors. Similarly, the efficacy of nutritional support to improve outcomes in many circumstances is unproven, but data are accumulating suggesting that there are measurable benefits from interventions to correct or prevent nutritional deficits.
PEM may occur as a consequence of inadequate intake alone (e.g., starvation) or in association with disease-activated physiological mechanisms that affect body metabolism, composition, and appetite (i.e., cachexia). In the former (primary caloric deficiency state), the body adapts by using fat stores while conserving protein and muscle, and the resulting physiological changes are often reversible with resumption of usual intake and activity. Cachexia is marked by an acute phase response that is associated with elevated inflammatory mediators (e.g., tumor necrosis factor-α [TNF-α] and interleukin-6) and increased protein and muscle degradation that may not be readily reversed by refeeding. Although cachexia is usually associated with specific chronic disease conditions (e.g., cancer, infection), this state may develop in older persons without obvious underlying disease. To some extent, these physiological changes maybe adaptive. Thus, caution is necessary when devising strategies to halt the lean body mass loss and functional decline that often accompanies cachexia.
Despite its apparent clinical importance, physician recognition of malnutrition is often lacking. Effective management of frail or ill older adults mandates an evaluation of nutritional status to better allow for early recognition of PEM and consideration of appropriate interventions. Assessment of nutritional status by standard anthropometric, biochemical, and immunological measures can be complex, as both nutrient intake and nonnutrition-related factors can affect these parameters. The use of such measurements (e.g., body mass index, skinfold thickness, muscle circumferences, serum concentrations of proteins, and lymphocyte counts) to detect the presence of poor nutrition is often advocated, but may not result in earlier or more effective intervention than can be achieved by a careful history and physical examination. The close monitoring of body weight, a readily obtainable measure that reflects imbalance between caloric intake and energy requirements, maybe the simplest and most reliable way to screen for malnutrition, particularly in the outpatient setting. Body weight should be recorded at all patient visits. Weight change should be expressed as a percentage of change from past to current weight, because proportional weight change helps account for variability in baseline weight and appears to be the most clinically relevant measure. Although weight gain caused by excessive energy intake is a common form of malnutrition, only undernutrition and weight loss caused by deficits in energy balance are considered here. Weight loss of 5% or more of usual body weight, a degree that has been associated with increased morbidity and mortality in the outpatient setting, should prompt investigation. Illness-related weight loss exceeding 10% of preillness weight is associated with functional decline and poor clinical outcomes. Weight loss of 15% to 20% or more of usual body weight implies severe malnutrition.
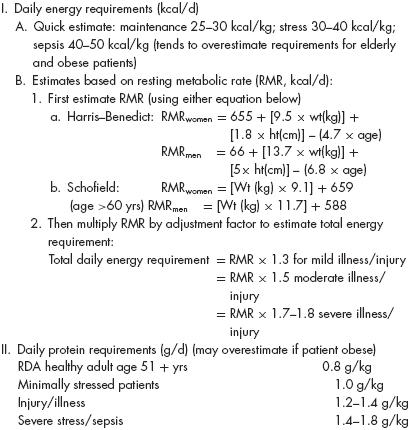
In the hospital setting, where acute illness or injury often coexists with inadequate intake, alterations in nutritional parameters associated with PEM may develop rapidly. Elevated levels of inflammatory mediators appear to be responsible for the greater losses in lean body mass and the rapid declines in albumin that often accompany PEM in physiologically stressed patients. While serial weight measures remain clinically relevant, early detection and correction of PEM in acutely ill hospitalized patients is enhanced by determination of dietary intake relative to metabolic requirements. Although registered dietitians often provide this information, physicians should routinely monitor dietary intake and can readily estimate caloric and protein requirements using formulas presented in Table 40-2. Biochemical and immunological measures (e.g., albumin, prealbumin, transferrin, and lymphocyte counts) are useful adjuncts in the assessment of nutritional status and can provide prognostic information, but their lack of specificity limits their utility as markers of PEM. Anthropometric measures of fat stores (skinfolds) and muscle mass (midarm muscle area) may help in the assessment of PEM, but clinicians are generally not well versed in obtaining these measures and interrater variability can be high. Although less sensitive, clinical evaluation for loss of skin turgor and the presence of atrophy in hand interosseous or head temporalis muscles can help assess for losses in subcutaneous fat and muscle mass. Because all of these parameters maybe affected by nonnutrition-related factors, an effective assessment of nutritional status requires synthesis of information provided from the dietary history, physical examination, and biochemical data. There are no definitive criteria for classifying degrees of PEM. However, when weight loss exceeds 20% of premorbid weight, serum albumin is less than 21 g/L, transferrin is less than 1 g/L, and total lymphocyte count is less than 800/μL, PEM is generally considered to be severe.
Initial management of patients with PEM and/or weight loss should include a thorough evaluation to identify underlying causes, and if found, to aggressively attempt to correct potentially remediable factors. In the acute care setting, the cause(s) of malnutrition are often readily evident, although depression maybe a contributing factor that is frequently overlooked. In contrast, reasons for poor nutrition and weight loss among community-dwelling elderly persons maybe multiple and not as readily discernible. Data available from studies on the causes of involuntary weight loss suggests that depression, gastrointestinal (GI) maladies (most often peptic ulcer or motility disorders), and cancer are the three most common causes of weight loss in older adults (Table 40-3). In these studies, when cancer was the cause of weight loss, the diagnosis was rarely obscure. Most diagnoses were readily made after standard evaluations that included a careful history and physical examination and basic screening tests (urinalysis, complete blood count, serum electrolytes, renal, liver and thyroid function tests, stool hemoccults, and a chest radiograph), with additional tests only as directed by signs and symptoms. If this initial basic evaluation is unrevealing (as the data in Table 40-3 suggests will occur in around 25% of cases), it is best to enter a period of “watchful waiting” rather than pursue more extensive undirected testing. A diagnostic algorithm (Figure 40-1) focusing first on verifying actual weight loss (patients may inaccurately report a history of weight loss) and then on whether caloric intake is adequate can help guide an appropriate workup.
STUDY (STUDY SIZE) | ||||||
|---|---|---|---|---|---|---|
Marton et al. (N = 91) | Rabinovitz et al. (N = 154) | Huerta et al. (N = 50) | Lankisch et al. (N = 158) | Levine (N = 107) | Thompson et al. (N = 45) | |
Study population | 70% Inpatient | Inpatient | Inpatient | Inpatient | Outpatient | Outpatient |
Weight loss definition* | ≥5%/6mos. | ≥5%/not stated | ≥10%/6mos. | ≥5%/6mos. | ≥5%/6mos. | ≥7.5%/6mos. |
Mean age (range) | 59 ± 17† | 64 (27–88) | 59 (18–83) | 68 (27–92) | 62 (17–91) | 72 (63–83) |
Gender (% male) | 100% | 45% | 64% | 44% | 53% | 33% |
Diagnosis (n[%]) | ||||||
Neoplasm | 18 (19%) | 56 (36%) | 5 (10%) | 38 (24%) | 6 (6%) | 7 (16%) |
Gastrointestinal | 13 (14%) | 26 (17%) | 9 (18%) | 30 (19%) | 6 (6%) | 5 (11%) |
Psychiatric | 8 (9%) | 16 (10%) | 21 (42%) | 17 (11%) | 24 (22%) | 8 (18%) |
Endocrine | 4 (4%) | 6 (4%) | 5 (10%) | 18 (11%) | 5 (5%) | 4 (9%) |
Cardiopulmonary | 13 (14%) | — | 1 (2%) | 16 (10%) | 9 (9%) | — |
Other medical diagnoses‡ | 16 (18%) | 14 (9%) | 4 (8%) | 13 (8%) | 17 (16%) | 10 (22%) |
Unknown | 24 (26%) | 36 (23%) | 5 (10%) | 26 (16%) | 38 (36%) | 11 (24%) |
Older persons who are not meeting their protein and caloric requirements through oral intake should be considered for nutritional support. Table 40-4 outlines approaches to nutritional support and factors to consider in deciding whether to pursue specific interventions. The urgency for nutritional interventions relates to the degree of protein-calorie depletion at the time of diagnosis coupled with the expected magnitude and duration of inadequate nutrition. In the hospital setting, clinicians must consider that patients may have been suffering from PEM for some time prior to admission. Therefore, avoidance of delays in instituting appropriate nutritional support while waiting for improved intake is desirable. One approach is to intervene after a period of 5 to 7 days of severely limited intake, or for weight loss more than 10% of preillness weight in hospitalized patients. However, attempts should be made to prevent PEM rather than wait for this degree of PEM to develop because weight loss and undernutrition are associated with worse clinical outcomes and recovery of lost lean body mass is often difficult. This is particularly important in severe stress states (e.g., sepsis, major injury) where protein catabolism can lead to losses of lean body mass that approach 0.6 kg/d. In support of early intervention when the development of PEM is likely, a trial of enteral nutrition (EN) among patients with major injury found that early (within 24 hours) enteral feeding had clinical benefits over tube feeding started later in the course of hospitalization.
AVAILABLE INTERVENTIONS | FACTORS TO CONSIDER |
|---|---|
Enhance oral intake | Degree of baseline protein-calorie depletion |
Frequent meals, snacks | Current intake relative to requirements |
Enhance food flavor | Expected duration of |
Provide favorite foods | inadequate nutrition |
Protein-calorie supplements | Effect of intervention on clinical outcomes |
Multivitamins | Potential benefits |
Appetite stimulants | Potential adverse effects |
Anabolic agents | Potential for reversibility |
Enteral nutrition | Quality of life |
Parenteral nutrition | Patient/family care preferences |
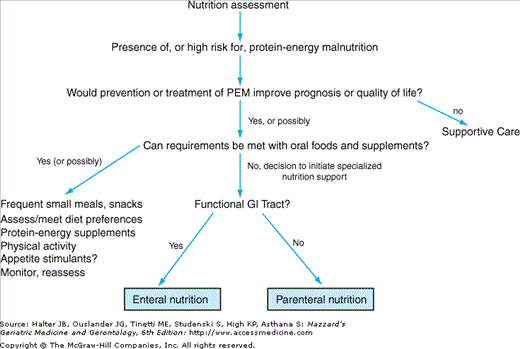
The effect of the planned intervention on the patient’s quality and/or quantity of life should be addressed before proceeding with nutritional support. Although nutritional support interventions may improve weight and other nutritional parameters, evidence demonstrating their ability to improve clinical outcomes is still limited, particularly when PEM is associated with serious (e.g., critically ill intensive care unit patients) or irreversible underlying disease (e.g., cancer). While most agree that efforts to improve nutrition, even in patients with serious underlying disease, are warranted, late in the course of disease appropriate palliative care may include, if not mandate, discontinuing such efforts. Determination of the care preferences of the patient and family is a critical component of the decision-making process. Patient and family counseling prior to implementing nutritional support should include a review of the interventions being considered and their potential for adverse, as well as beneficial, effects. Figure 40-2 shows an algorithm to help guide nutritional support decisions.
Although it is uncommon for elderly persons with PEM to be able to increase their food consumption sufficiently to correct their nutritional deficits, short trials of strategies to improve voluntary intake are reasonable for stable patients with mild PEM (no definitive criteria exist, but parameters consistent with mild-moderate PEM include weight 85–90% of premorbid weight, albumin 25–30 g/L, transferrin 1.5–2.0 g/L, total lymphocyte count 800 to 1200/μL). As previously outlined, underlying causative or contributing condition(s) to PEM should be considered and addressed. Strategies to help overcome anorexia and improve oral intake include assessing and meeting food preferences, providing frequent small meals and snacks, use of flavor-enhanced foods, and providing favorite high-calorie foods (i.e., sweets). Dietitians may aid greatly in these efforts.
High-calorie snacks and oral nutritionally complete supplements are generally recommended. Randomized trials provide some evidence that oral nutritional supplements can improve dietary intake, prevent or lessen weight loss, improve measures of immune function, and improve health outcomes of older adults with, or at high risk for, malnutrition. Higher-calorie “plus” variety nutritional supplements (1.5–2.0 kcal/mL) are preferred over standard (1 kcal/mL) formulas. Costs are only slightly higher, and because they deliver higher calorie content per milliliter ingested, patients need not drink as much volume to improve caloric intake. Supplements should be provided between, rather than with, meals as this appears to result in less compensatory decreases in food intake at mealtime, thereby more effectively increasing total daily caloric intake. However, even if total caloric intake is only marginally improved, the provision of energy from nutritionally dense supplement sources maybe beneficial because of improved micronutrient intake.
A standard multivitamin supplement should also be considered for all older adults with poor intake. Evidence suggests that improving micronutrition with multivitamins, particularly in malnourished or at-risk persons, can improve clinical outcomes (e.g., infection rates). Increased physical activity is an important adjunct that can improve appetite and sense of well-being, thereby leading to improved caloric intake, micronutrient, and functional status.
Pharmacologic approaches to stimulate appetite and promote weight gain maybe considered on an individual basis with the knowledge that the few agents (megestrol acetate [MA], dronabinol, cyproheptadine) that can improve intake in some patient populations (e.g., cancer, human immunodeficiency virus [HIV], anorexia nervosa) have not been shown to be effective in older adults and have the potential to cause important side effects. Further, the weight gain that has been observed with these agents has usually been small, disproportionately fat mass, and not associated with improved function, quality of life, or decreased morbidity and mortality. MA and dronabinol have shown, at best, limited promise in relatively small studies conducted in nursing home patients. Also of note, in one small nursing home trial, MA did not increase food intake unless combined with increased mealtime feeding assistance. A head-to-head comparison trial in cancer patients found MA to be more effective than dronabinol in improving appetite and promoting weight gain. MA’s mechanism of action may involve suppression of inflammatory cytokines (e.g., interleukin-6 and TNF-α), consistent with the observation that this agent appears most effective in persons with elevated cytokine levels (e.g., cancer or acquired immunodeficiency syndrome [AIDS] patients). If initiated, MA may not have a demonstrable effect on appetite for several weeks, but if no effect is seen by 8 weeks, MA should probably be discontinued. If positive effects are demonstrated without significant side effects, MA maybe continued for up to 12 weeks. Potential side effects include fluid retention, nausea, glucose intolerance, and venous thromboembolism. Treatment for more than 8 to 12 weeks can suppress the adrenal–pituitary axis, resulting in adrenal insufficiency and insufficient response to acute stressors. MA also appears to blunt the beneficial effects of progressive resistance exercise, consistent with an undesirable glucocorticoid-like catabolic effect. Only very preliminary data exists for dronabinol use in older adults. Although potential benefits of this agent include positive effects on pain, mood, and nausea, as well as appetite, further study is needed to clarify its efficacy and safety in this population. Delirium is the major side effect of concern from dronabinol.
If depression is felt to be a likely, or possible, contributing factor to poor intake, a trial of therapy is usually warranted. Selective serotonin reuptake inhibitors (SSRIs) are first-line antidepressant agents and improve appetite by improving depression. Mirtazapine and tricyclic antidepressants (e.g., nortriptyline) have been claimed to increase appetite independent of effects on mood. However, one retrospective study did not find a difference between the effects of SSRIs versus tricylcic antidepressants on weight change.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree