Familial adenomatosis polyposis
PTEN hamartoma tumor (Cowden’s)
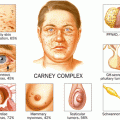
Carney complex
type 1
Pathognomonic criteria
> 100 colorectal adenomatous polyps
Mucocutaneous lesions and cerebellar tumors
Pigmented skin lesions, i.e., lentigines, cutaneous, and/or mucosal
Major manifestations
–
Breast, endometrial, and thyroid cancer; macrocephaly
Blue nevi, pigmented nodular adrenals, cardiac myxomas
Minor
Extracolonic polyps, congenital hypertrophy of retinal pigment epithelium; soft tissue tumors; desmoids; osteomas
Fibrocystic breast disease, mental retardation, gastrointestinal hamartomas, lipomas; fibromas, renal cell carcinomas, uterine fibroids
Melanotic schawannomas; adrenal, hepatocellular, or pancreatic cancers; pituitary adenomas
Gene
APC (80 %)
PTEN (80 %)
PRKAR1A (70 %)
Prevalence of thyroid disease:
Benign
40 %
Up to 75 %
Up to 75 %
Hashimoto’s thyroiditis in 40 %
Cancer
0.4–12 %
35 %
< 5 %
Cancer types
CMV-PTC 63 %
PTC 50 %
PTC
FV-PTC 25 %
FV-PTC 28 %
FTC
PTC 12 %
FTC 14 %
Presentation
Possibly more likely in women
Thyroid cancer is earliest presentation
–
Surveillance
Thyroid ultrasound, neck exam, and TSH at diagnosis, then yearly
Treatment
Total thyroidectomy when surgery indicated
FNMTC can also be diagnosed in the absence of an identified predisposition syndrome or history of radiation exposure, and is defined by DTC in at least two first-degree relatives [21]. In a population-level Nordic study, a threefold elevated risk of thyroid cancer was observed even if a single first-degree relative was diagnosed with either PTC or FTC. The risks were more pronounced if the relative was diagnosed at a young age although there was a trend towards concordant age at DTC diagnosis [22]. A number of candidate loci have been identified including MNG1, TCO, fPTC/PRN, NMTC1, FTEN, and FOXE1 [23]. Nonsyndromic FNMTC may be associated with earlier age onset, multifocality, extrathyroidal extension, and lymph node metastasis. Prophylactic thyroidectomy is also not necessary although neck ultrasound is recommended at age 20 or 5–10 years before the proband was diagnosed, and total thyroidectomy is typically performed when surgical intervention is indicated [21].
Genetics of Sporadic DTC
The majority of DTC are sporadic, and the most common genetic alterations implicated in thyroid carcinogenesis involve activating mutations in the mitogen-activated protein kinase (MAPK) and PI3K-AKT pathways that lead to downstream upregulation of tumor-promoting and cancer progression genes [24]. The type of mutation correlates to histologic subtype (Table 2) [24, 25]. For example, FTC are more likely to have mutations in the RAS and PTEN genes while BRAF V600E and RET/PTC rearrangements are more common in conventional PTC . Interestingly, follicular-variant PTC which has morphologic features more consistent with PTC but biologic behavior that is often similar to FTC, shares gene alterations with both histologic types.
Table 2
Correlation between gene alteration and differentiated thyroid cancer subtype
Genetic alteration | Associated DTC |
|---|---|
Mutations | |
BRAF V600E | Conventional PTC |
Tall-cell-variant PTC | |
Follicular-variant PTC, unencapsulated | |
BRAF K601E | Follicular-variant PTC, encapsulated |
RAS | Follicular adenoma |
Conventional PTC | |
Follicular-variant PTC | |
FTC | |
OTC | |
PTEN | FTC |
OTC | |
Conventional PTC (rare) | |
TSHR | FTC |
Follicular-variant PTC | |
P53 | Conventional PTC |
OTC | |
PIK3CA | FTC |
Rearrangements | |
RET/PTC | Conventional PTC |
Follicular-variant PTC (rare) | |
PAX8-PPARg | Follicular-variant PTC |
FTC | |
BRAF V600E is the most common gene alteration in thyroid carcinogenesis, and can be associated with up to 40–50 % of conventional PTC. BRAF mutations may correlate with aggressive tumor characteristics such as extrathyroidal extension, advanced tumor stage at presentation, and lymph node or distant metastases, and may be an independent, age-related predictor of tumor recurrence [26, 27]. In a cohort study, persistent/recurrent disease was most likely in PTC patients who had BRAF V600E-positive tumors and who were age ≥ 65 years [28]. Xing et al. evaluated a multi-institutional and retrospective series of 1840 PTC patients with median follow-up of 33 months, and observed an association between BRAF V600E and increased disease-specific mortality. However, in multivariate analysis, the effects of BRAF did not appear to be independent of extent of disease at presentation including presence of lymph node metastasis, extrathyroidal extension, and distant metastasis [29].
RAS has a key role in signal transduction from tyrosine kinase and G-protein-coupled receptors to effectors of both the MAPK and PI3K-AKT pathways. Point mutations cause increased affinity of RAS to guanosine triphosphate (GTP) or inhibit the autocatalytic GTPase function, and both cause constitutive activation of downstream pathways [30]. RAS mutations are the most common gene alterations identified in indeterminate biopsy specimens, and can include point mutations in N-, K-, and H-RAS hotspots in codons 12/13 and 61 [31]. All three mutant isoforms have been identified in up to 48 % of benign follicular adenomas, 50–60 % of FTC, and less frequently (20 %) in PTC [30]. Although most RAS-associated thyroid cancers are indolent, RAS mutations have also been identified in medullary, poorly differentiated, and anaplastic thyroid cancers.
RET rearrangements in PTC have been well documented and more than 10 different types of translocations have been described that are identified in 10–20 % of PTC [32, 33]. Typically, the 3′ tyrosine kinase portion of the RET gene fuses with the 5′ of a different gene resulting in a ligand-independent dimerization and constitutive activation of effector genes in both the MAPK and PI3-AKT pathways. A higher incidence of RET/PTC rearrangements are seen in PTC patients with a previous history of radiation exposure (50–80 %) and in younger patients (40–70 %) [34, 35]. The two most common fusion proteins are RET/PTC1 and RET/PTC3; RET/PTC1-positive tumors demonstrate either classic papillary architecture or diffuse sclerosing features, while RET/PTC3 is associated with solid-variant PTC. All of the RET/PTC tumor subtypes have a higher rate of lymph node metastases [36].
Less commonly identified gene alterations in PTC and FTC include BRAF K601E, thyroid-stimulating hormone receptor ( TSHR), and somatic PTEN mutations in addition to the PAX8/PPARγ rearrangement (Table 2). A shift in diagnosed thyroid cancer histologies and molecular profiles was observed in an evaluation of thyroid cancers over four decades at a single institution [37]. In addition to an increased median age at diagnosis, the proportion of classic PTC was observed to decrease over time. Three interesting temporal trends in DTC molecular profiles were observed: (1) The percentage of BRAF V600E-positive PTC remained stable although the proportion of classic PTC with BRAF V600E mutations increased in the later decades, (2) the prevalence of RAS mutations in thyroid cancers increased, and (3) RET/PTC-positive PTC decreased over time suggesting that exposure to ionizing radiation may have a diminishing contribution to thyroid carcinogenesis [37].
Mitochondrial DNA mutations have been proposed as an etiology in formation of OTC. Mutations in GRIM-19, a regulatory gene involved in apoptosis and mitochondrial metabolism, have been identified in ~ 15 % of oncocytic or Hürthle cell variants of follicular and papillary thyroid carcinoma [38]. Point mutations in p53, RAS, and PTEN were identified in OTC after evaluation with next-generation sequencing, a method that allows parallel high-volume sequencing, that targets a panel of 284 hot-spot mutations previously reported in DTC [39]. However, 60 % of the studied OTC did not have any of the evaluated mutations and further study of the gene alterations that lead to OTC is still needed.
Accumulation of genetic alterations that lead to further dysregulation is one likely trigger for tumor progression, and multiple driver mutations can be found in recurrent/metastatic DTC [24, 39]. Another marker that is associated with aggressive DTC is mutation of the telomerase reverse transcriptase ( TERT) promoter. Telomerase activation is a marker of malignancy that allows continued cell replication and TERT promoter mutations have been identified in other malignancies. TERT promoter mutations in thyroid cancers were identified in 7–22 % of PTC and ~ 35 % of FTC, were often found in association with BRAF or RAS mutations, and were more likely in patients with histologically aggressive differentiated thyroid cancer [40, 41]. In a series of 469 thyroid cancer patients of whom 402 had differentiated thyroid cancer, Melo et al. [42] reported that TERT promoter mutations were independently associated with an increased risk of disease-specific mortality for both PTC and FTC patients. Other markers of aggressive thyroid cancer have often been synonymous with tumor dedifferentiation and these include mutations in p53 (25–30 %), PIK3CA (10–20 %), CTNNB1 (10–20 %), and AKT1 (5–10 %) [25] .
Clinical Manifestations
Most patients with DTC are asymptomatic . The most common presentation is a thyroid nodule found either on physical exam or incidentally on diagnostic imaging such as CT scan of the chest/neck or carotid duplex imaging. New-onset hypothyroidism may also be an indicator of malignancy, and a careful cervical and thyroid examination should be performed. A clinical history of an enlarging thyroid mass increases the concern for malignancy. On physical exam, an immobile and firm thyroid nodule is also worrisome. Routine evaluation should also assess for dysphagia, positional dyspnea, orthopnea, anterior neck discomfort, hoarseness, tracheal deviation, lymphadenopathy, and the presence of contralateral thyroid nodules. Supine dyspnea that is relieved by positional change, a history of sleep apnea, and/or an inability to palpate the inferior aspect of the thyroid gland should raise concern for a substernal component .
Diagnosis
Neck ultrasound is the gold-standard imaging study used to evaluate thyroid nodule size and nodule characteristics . Suspicious sonographic features include solid consistency, taller-than-wide shape, marked hypoechogenecity, irregular border, intranodular hypervascularity, microcalcifications, and loss of the echogenic halo (Fig. 1a) [43]. Cervical lymphadenopathy should also always be concurrently evaluated on cervical ultrasound. Lymph nodes that are rounded, have microcalcifications, peripheral vascularization, loss of the fatty hilum, rounded shape, and cystic appearance are concerning and should undergo FNAB (Fig. 1b). A noncontrast CT scan of the neck can be considered if there is clinical concern for substernal extension particularly if the caudal extent of the thyroid gland is not palpable on physical exam as substernal extension below the aortic arch may require a partial sternal split for complete resection .
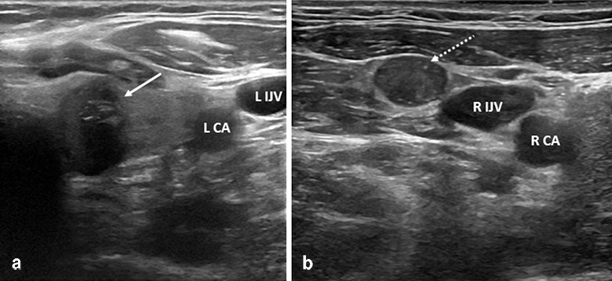

Fig. 1
Preoperative ultrasound images of a left thyroid nodule (a, white arrow) that has several suspicious sonographic features including taller-than-wide shape, marked hypoechogenecity, microcalcifications, and no well-defined halo. The patient also had a concerning right level 3 lymph node (b, dotted arrow) that was rounded and lacked a fatty hilum. Histology confirmed multifocal BRAF V600E-positive papillary thyroid cancer with metastatic disease in both the central compartment and right lateral neck
FNAB is the most sensitive initial diagnostic test and also guides subsequent clinical management. For reporting standardization, cytology results should be classified into one of the six-tiered Bethesda System for Reporting Thyroid Cytopathology categories: inadequate, benign, atypia or follicular lesion of undetermined significance, follicular neoplasm or suspicious for follicular neoplasm, suspicious for malignancy, or positive for malignancy [44]. Thyroidectomy is currently indicated for FNAB results in the indeterminate, positive for malignancy, or persistently inadequate categories. Diagnostic adjuncts may further identify if nodules with indeterminate cytology require surgery, and include (a) molecular testing for somatic mutations/rearrangements that are found in thyroid cancer [31, 45], (b) evaluation of gene expression profiling patterns that may identify nodules at lower risk for malignancy [46], or (c) identification of circulating TSHR messenger RNA (mRNA) that may be a marker of malignancy [47]. Patients with risk factors for thyroid cancer who have a dominant (> 1 cm) thyroid nodule, symptomatic thyromegaly, tracheal compression, and/or substernal goiter that are unable to be evaluated completely with FNAB or ultrasound (US) should also be considered for thyroidectomy .
Large nodules ( ≥ 4 cm) may have a higher risk of malignancy (7–35 %) in addition to a higher likelihood of false-negative benign FNAB results (0.9–20 %) [48], and the inability to accurately exclude malignancy in large nodules may be considered another indication for surgery. Banks and colleagues developed a predictor model to aid in the diagnosis of suspicious or indeterminate FNA samples and reported that patient age, nodule size (nodules < 1.5 cm and larger nodules ≥ 3 cm were associated with increased risk), and cytopathology (suspicious for papillary thyroid carcinoma had the greatest risk) were all significant predictors of thyroid cancer. These three variables remained significant on multivariate analysis and, in combination, were predictive with 74 % accuracy. The model was then prospectively validated in a separate cohort of 135 thyroidectomy patients, and was able to predict thyroid cancer in ROC analysis with an AUC of 0.85 [49] .
Surgical Management
Although PTC can be readily diagnosed on FNAB, the diagnoses of FTC and OTC require further histologic examination of the entire nodule and its capsule in relation to the adjacent thyroid lobe tissue . The decision for initial extent and conduct of thyroidectomy depends on the degree of preoperative concern for DTC, size of the presumptive malignancy, and other clinical features.
Among low-risk DTC patients who undergo lobectomy only, studies suggest a higher rate of local (up to 30 %) and contralateral lobe (5–10 %) recurrence; however, effects on long-term survival are less clear [50]. For any DTC patient > 45 years who also has extrathyroidal extension and/or palpable lymph node metastases (i.e., American Joint Committee on Cancer (AJCC) TNM stage III disease), total thyroidectomy versus thyroid lobectomy improves cause-specific mortality at 30 years (20 vs. 39 %) [51]. In a study of > 50,000 PTC patients from the National Cancer Data Base, total thyroidectomy compared to lobectomy for PTC ≥ 1 cm was associated with statistically significant improvement in 10-year local recurrence rates (7.7 vs. 9.8 %) and 10-year survival (98.4 vs. 97.1 %). For PTC < 1 cm, the extent of thyroidectomy made no difference in recurrence or survival but the findings of this study are limited by heterogeneity in PTC pathologic subtypes, use of radioactive iodine (RAI) ablation, and variable management with TSH suppression [52] .
Thyroidectomy for DTC ≥ 1 cm Diagnosed by Fine-Needle Aspiration
As mentioned previously, DTC diagnosed preoperatively is typically PTC, and thyroidectomy is the initial treatment. The false-negative rate of FNAB that is positive for malignancy is low (1–2 %) and patients should be counseled preoperatively about the possibility of false-positive results. Total thyroidectomy or near-total thyroidectomy (which leaves £ 50 mg of tissue at the ligament of Berry) is considered the procedure of choice when the tumor measures ≥ 1 cm on preoperative US. Among experienced thyroid surgeons, initial total thyroidectomy is usually associated with a < 1 % risk of recurrent laryngeal nerve injury and a < 1 % risk of permanent hypoparathyroidism [53] .
Thyroidectomy for DTC < 1 cm Diagnosed by Fine-Needle Aspiration
FNAB for thyroid nodules < 1 cm is typically not performed unless suspicious sonographic features are present. If diagnosed preoperatively, the management of small, papillary thyroid microcarcinomas (PTMC) remains controversial not just in determining the extent of surgery but also in deciding if surgical management should be pursued. Although most studies suggest that PTMC is generally associated with an excellent long-term survival regardless of surgical procedure, there is a small but real percentage of patients who develop lymph node metastasis (up to 35 %) and distant metastasis (< 1 %). In one meta-analysis, recurrent disease from PTMC was associated with multifocality, “nonincidental” disease, age < 45 years, and the presence of lymph node disease at presentation [54]. In the absence of these factors, thyroid lobectomy is considered adequate treatment for unifocal PTMC .
Nonoperative management has also been studied by Ito et al. [55]. In a series of 340 patients with PTMC who had observation instead of thyroidectomy, enlargement of the tumor more than 3 mm and lymph node metastasis occurred at 10-year follow-up in 15.9 and 3.4 % of the patients, respectively. When compared to patients with PTMC who received immediate thyroidectomy , the rate of lymph node recurrence was the same. Furthermore, most patients who had nonoperative treatment had stable disease even at 10-year follow-up [55]. Nonoperative treatment may be considered for selected patients with intrathyroidal tumors without evidence of lymph node metastasis, although studies with longer follow-up are still needed to determine the natural history of in situ PTMC .
Diagnostic Thyroidectomy
DTC is often first diagnosed on postoperative histology and follicular-variant PTC is now the most common histologic malignancy diagnosed after diagnostic thyroidectomy. For FNAB results in the indeterminate, persistently nondiagnostic, or suspicious categories, diagnostic thyroid lobectomy and isthmusectomy at a minimum is indicated and is associated with histologic DTC in 20 , 5–10, and 50% of cases, respectively [56]. The minimum extent of initial diagnostic thyroidectomy is ipsilateral complete lobectomy, and isthmusectomy as the thyroid isthmus is an important anatomic and surgical margin intraoperatively. Partial thyroid lobectomy is an outmoded operation that puts the ipsilateral recurrent laryngeal nerve at unnecessary risk should reoperation be required. Since lobectomy and isthmusthectomy is associated with a 25–40 % chance of surgical hypothyroidism requiring chronic replacement levothyroxine therapy, patients should be counseled about this possibility preoperatively.
Extent of Initial Thyroidectomy
The extent of initial thyroidectomy is influenced by a number of clinical factors. For patients with FNAB-proven DTC < 1 cm in size or who require diagnostic thyroidectomy , the evidence to date supports initial total thyroidectomy for patients who have: a history of radiation exposure, diagnosed hypothyroidism, a family history of DTC, suspicious sonographic nodule features, a contralateral macronodule (>1 cm), or evidence of lymph node or distant metastasis. A preoperative tumor mutation profile that may be more likely identified in DTC with aggressive histologic features such as TERT or BRAF V600E may also be an indication for initial total thyroidectomy .
Completion Thyroidectomy
For PTC, completion total thyroidectomy (also termed reoperative contralateral lobectomy) to a thyroid remnant ≤ 1 gm is recommended if total thyroidectomy would have been performed had the diagnosis been known preoperatively, i.e., the tumor is ≥ 1 cm, high-risk histologic subtype (such as tall cell, sclerosing, or columnar variant), multifocal, nonencapsulated, and/or with apparent nodal involvement [57]. Widely invasive FTC is associated with a worse prognosis and such patients should undergo total thyroidectomy. In the absence of these factors, thyroid lobectomy is usually considered adequate treatment for unifocal PTC or FTC < 1 cm. Because OTC can be multifocal and does not take up RAI as effectively, many support an aggressive surgical approach with total thyroidectomy for all OTC regardless of size .
Lymphadenectomy
Although cervical lymph node involvement is common in DTC and ranges from 20 to 90 % depending on the sensitivity of the detection method, the prognostic significance of lymph node metastasis is controversial. Nodal disease is clinically apparent in < 10 % of patients with PTC or OTC, and most patients have occult micrometastases which likely have little to no prognostic significance for most patients as micrometastatic disease responds well to radioiodine therapy. Metastatic PTC documented in cervical nodes is associated with a risk of local recurrence; however, whether there is also an association with long-term PTC survival has not been clearly demonstrated. In a large Surveillance, Epidemiology, and End Results (SEER) database study evaluating patient outcomes, lymph node metastasis has been shown to be a particularly poor prognostic indicator among FTC and older (> 45 years) PTC patients [58]. Metachronous lymph node metastasis is associated with a higher rate of recurrence and disease-specific mortality and develops in 5–10 % of patients with PTC after thyroidectomy and RAI ablation [59, 60]. In contrast, metachronous nodal disease develops in up to 25 % of patients after thyroidectomy for OTC [61]. Synchronous and metachronous lymph node metastases are rare in FTC and are an indicator of poor prognosis [62]. Whether synchronous or metachronous, the presence of clinically apparent lymph node disease prompts surgical local control to prevent complications related to airway and venous obstruction .
In the preoperative evaluation of DTC, ultrasound is the best test currently used to identify nodal metastases. FNAB with or without thyroglobulin measurement of the aspirate is recommended when clinical or sonographic evidence of nodal metastasis is present. When cervical metastasis is confirmed, a therapeutic function-preserving and compartment-oriented lymphadenectomy is recommended to decrease local recurrence and limit the need for potentially morbid reoperative neck surgery.
DTC, particularly PTC, usually first metastasizes to the central compartment (Level VI) [63]. The central compartment is defined by the boundaries of the hyoid bone (superiorly), carotid arteries (laterally), superficial layer of the deep cervical fascia (anteriorly), and deep layer of the deep cervical fascia (posteriorly). The inferior boundary is defined on the right by the innominate artery and on the left by the equivalent plane. A unilateral central compartment lymph node dissection includes the prelaryngeal (Delphian), pretracheal, in addition to either the right or left paratracheal lymph nodes while a bilateral dissection includes both paratracheal nodal basins. While the data on whether or not prophylactic central neck dissection improves survival is conflicting, routine prophylactic clearance of central compartment nodal tissue has been demonstrated to lower postoperative thyroglobulin levels at 6 months and may decrease recurrence rates in meta-analysis [64]. Prophylactic central neck dissection may also lead to more accurate staging and risk stratification and allow for a selective approach to RAI ablation [65] . However, the risk of hypoparathyroidism appears to be more frequent after thyroidectomy with central compartment neck dissection than after thyroidectomy alone and this risk, as well as the risk of recurrent laryngeal nerve injury, should be considered when deciding whether to perform prophylactic central neck dissection [64].
The lateral compartment is defined by the boundaries of the internal jugular vein (medially), trapezoid muscle (laterally), subclavian vein (inferiorly), and hypoglossal nerve (superiorly). The lymph nodes within the lateral compartment are further separated by anatomic boundaries defining levels I–V. The most common levels involved with metastatic thyroid cancer are II–IV. In the absence of gross involvement, a modified neck dissection which preserves the internal jugular vein, sternocleidomastoid, spinal accessory, and phrenic nerves, is usually performed for metastatic DTC. Prophylactic lateral neck dissection has never been shown to improve long-term outcomes and is not recommended for DTC patients .
Staging
A number of staging systems exist for DTC that incorporate clinical and histologic features including MACIS (metastasis, age, completeness of resection, invasion, size), AGES (age, grade, extent, size), and AMES (age, metastasis, extent, size) . The most commonly used is the AJCC TNM staging system currently in its seventh edition which takes into account patient age, tumor size and degree of extrathyroidal extension, nodal metastasis, and distant metastasis (Table 3) [66]. Advanced stage disease is typically classified as AJCC TNM stage III (characterized by large tumor size > 4 cm, extrathyroidal tumor extension, and/or central compartment lymph node metastasis) or stage IV (characterized by gross tumor involvement to the adjacent structures, lateral lymph node and/or distant metastasis). Age at diagnosis has a significant effect on prognosis, and elderly patients have a higher risk of disease-related mortality rate compared to younger patients. Unfortunately, older patients present with more aggressive disease with an increased likelihood of extrathyroidal extension, and lymph node and distant metastases, and their cancers tend to be less iodine-avid [67] . In contrast, patients younger than 45 years of age uniformly have an excellent prognosis and even in the presence of distant metastatic disease cannot have higher than stage II disease. All thyroid clinical scoring systems are imprecise and some patients who are stratified into low-risk groups may still be at risk for disease-related mortality [10].
Table 3
American Joint Committee on Cancer (AJCC) 7th Edition, TNM Staging for DTC [66]. (Used with permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original and primary source for this information is the AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer Science + Business Media)
Tumor | ||
Tx Cannot assess | – | – |
T0 No evidence of tumor | – | – |
T1 Size £ 2 cm | – | – |
T1a Size £ 1 cm | – | – |
T1b Size 1–2 cm | – | – |
T2 Size 2.1–4 cm | – | – |
T3 > 4 cm, or minimal extrathyroidal extension | ||
T4a Extends beyond capsule to subcutaneous soft tissues, larynx, trachea, esophagus, recurrent laryngeal nerve or recurrent laryngeal nerve | ||
T4b Invades prevertebral fascia, encases major vessels | ||
Nodal disease
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||