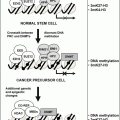
Date approved
Drug
Place
Disease
May 2003
Bortezomib
USA (FDA)
Multiple myeloma
December 2003
Thalidomide
Australia
Multiple myeloma
February 2004
Bevacizumaba
USA (FDA)
Colorectal cancer
November 2004
Erlotinib
USA (FDA)
Lung cancer
December 2004
Bevacizumaba
Switzerland
Colorectal cancer
December 2004
Macugena
USA (FDA)
Macular degeneration
January 2005
Bevacizumaba
EMEA
Colorectal cancer
September 2005
Endostatina
China
Lung cancer
December 2005
Sorafeniba
USA (FDA)
Kidney cancer
December 2005
Revlimid
USA (FDA)
Myelodysplastic syndrome
January 2006
Sunitiniba
USA (FDA)
Gastric (GIST), kidney cancer
June 2006
Lucentis
USA (FDA)
Macular degeneration
October 2006
Bevacizumaba
USA (FDA)
Lung cancer
Oxygen limitation is central in regulating angiogenesis, glucose metabolism, survival, and tumor growth [2]. A major key factor is the hypoxia-inducible factor (HIF) which is required transcriptional factor in nutrient stress signaling. HIF is a pleiotropic factor that controls the expression of VEGF-A and angiopoietin-2 [14]. In the nucleus, HIF links the hypoxia-response-elements that control oxygen tension via oxidizing enzymes and hydroxylases. Moreover, HIF also transcriptionally regulates the expression of MET promoter that is a key regulator of invasive growth by driving cell motility and metastasis [2].
Nitric oxide (NO) is a multifunctional gaseous molecule and a highly reactive free radical that regulates several vascular functions, being capable to mediate VEGF and angiopoietin-1-induced angiogenesis in vivo [15]. Inhibition of NO signaling is another potential antiangiogenic therapeutic strategy. A number of compounds interfere with NO production, such as cavtratin, a caveolin-1 derived peptide, L-NNa, and NO-donating nonsteroidal anti-inflammatory drugs (NSAIDs) [16–18].
More than 50 antiangiogenic agents with different mechanisms of action have been discovered up to now. They include tyrosine kinase inhibitors (TKIs), monoclonal antibodies, small molecule inhibitors and transcription inhibitors. Recently, nanobodies have been introduced into the antiangiogenic armamentarium.
39.2 Anti-VEGF Targeted Therapies
39.2.1 Bevacizumab
Bevacizumab is a humanized monoclonal antibody against VEGF. In February 2004, bevacizumab received the Food and Drug Authorization (FDA) approval for first-line therapy of colorectal cancer (CRC) in combination with an IFL-based (irinotecan, fluorouracil and leucovorin) regimen. In CRC, a randomized multicenter study evaluating the clinical benefits of bevacizumab combined with a bolus IFL in front-line treatment of CRC showed an advantage in median duration of overall survival, median duration of progression-free survival (PFS), response rate (RR) and median duration of response [19]. The most significant clinical toxicities reported in bevacizumab-treated patients were thrombosis, hypertension, proteinuria, and bleeding. In addition, gastrointestinal perforation (1.5 %) as well as an increase in diarrhea, leukopenia, and hypertension was also described. Results from the Three Regimens of Eloxatin Evaluation (TREE)-2 trial study in patients with metastatic CRC showed that the addition of bevacizumab to an oxaliplatin/fluorouracil regimen in first-line therapy improves RR and time to progression (TTP) with acceptable tolerability, and no unexpected toxicity [20]. Several other trials studying the clinical benefits of bevacizumab combined with different anti-EGFR agents, such as erlotinib, panitumumab, or cetuximab are ongoing.
The clinical utility of bevacizumab in metastatic RCC was investigated in a randomized phase II trial in which 116 patients with metastatic clear-cell RCC refractory to immunotherapy were randomized to receive placebo, low-dose (3 mg/kg) bevacizumab, or high-dose (10 mg/kg) bevacizumab given intravenously every 2 weeks. Patients treated with bevacizumab experienced a significant prolongation of TTP compared with the placebo group [21]. There were four partial responses (10 % ORR), all in the high-dose bevacizumab arm. There were no life threatening toxicities or deaths attributable to bevacizumab. Common toxicity included hypertension and proteinuria, more commonly seen in the high-dose bevacizumab arm. All side effects were reversible with cessation of therapy. Grade 1 or 2 hemoptysis was observed in two patients receiving bevacizumab and two patients receiving placebo. No thromboembolic events were reported in any arm.
In a double-blind phase II trial comparing bevacizumab plus erlotinib versus bevacizumab plus placebo, data showed that the combination was safe and well tolerated, although adding erlotinib did not improve efficacy [22]. However, PFS of 8.5 months observed in patients treated with bevacizumab [23] appears to be more favorable than what is reported with the use of IFN-α, suggesting a potential clinical benefit of bevacizumab in RCC. Given the promising activity of bevacizumab, several clinical trials investigating the clinical advantages of bevacizumab with IFN-α2b therapy (CALGB 90206; n = 600) (Rini = 72) or in addition to erlotinib plus imatinib are currently in progress [24].
The addiction of bevacizumab to carboplatin/paclitaxel regimen caused a higher RR (31.5 % in the high-dose arm versus 18.8 %) and longer median TTP (7.4 months in the high-dose arm versus 4.2 months) than chemotherapy alone in NSCLC patients [25]. Bevacizumab was generally well tolerated. However, severe haemoptysis episodes among patients with squamous cell histology and central tumor mass were observed. Another study evaluated the clinical benefits of bevacizumab with the carboplatin/paclitaxel regimen as first-line therapy in 842 patients with NSCLC. This US cooperative group phase III trial (E4599) reported a higher RR, longer PFS and increased survival in the bevacizumab/chemotherapy arm compared with the chemotherapy-alone arm [26]. In a trial combining bevacizumab with erlotinib in patients with recurrent NSCLC, preliminary data showed a promising antitumor activity, with partial response achieved in 20 % of patients and stable disease in 65 % [27]. The most common adverse events reported ranged from mild-to-moderate and included rash, diarrhea and proteinuria. Bevacizumab in combination with erlotinib is still under investigation in recurrent or refractory NSCLC [28].
A phase II study assessed the combination of bevacizumab plus gemcitabine in 52 patients with stage IV pancreatic cancer [29]. The objective RR was 21 %, the median PFS 5.4 months and the 6-month OS of 77 %. Adverse events included hypertension, thrombosis, and bleeding episodes. The promising efficacy prompted two ongoing trials: the European phase III trial (BO17706) and the US Cooperative Group phase III trial (CALGB 80303), investigating both the therapeutic benefits of bevacizumab when added to gemcitabine alone or with erlotinib.
Zhu et al. reported encouraging results from a phase II study investigating the therapeutic benefits of bevacizumab combined with a gemcitabine and oxaliplatin regimen in patients with advanced hepatocellular carcinoma [30]. Data suggested that this combination was generally well tolerated, with the most common grade 3–4 adverse events being fatigue, transient elevation of transaminases, nausea/vomiting and hypertension. Although no patients achieved a complete response, the overall response rate was 20 % and the rate of PFS at 6 months 48 %.
In a phase III metastatic breast cancer (mBC) trial, the addition of bevacizumab to capecitabine in a cohort of taxane-refractory and-anthracycline-refractory patients failed to show an improvement in PFS and OS [31]. The addition of bevacizumab to a carboplatin/albumin-bound form of paclitaxel and trastuzumab regimen showed promising antitumor activity among patients with HER2-positive mBC [32]. Of nine evaluable patients, eight achieved a major clinical response. The clinical activity of bevacizumab alone or in combination is being examined in ovarian cancer, recurrent cervical cancer, hormone-refractory prostate cancer, refractory or relapsed AML and malignant melanoma, as well as head and neck cancer. Bevacizumab is also being evaluated as adjuvant treatment of CRC in combination with different regimens including FOLFOX4, FOLFOX6, or XELOX (capecitabine/oxaliplatin) in the National Surgical Adjuvant Breast and Bowel Project (NSABP) C08 trial, the AVANT (AVastin adjuvANT) trial, and the Intergroup Rectal Adjuvant trial [33].
39.2.2 HuMV833
HuMV833 is a humanized monoclonal antibody that recognizes VEGF121 and VEGF165 isoforms. Results from a phase I trial showed that HuMV833 was safe and well tolerated in patients with solid tumors [34]. Infusions were well tolerated without any grade 3 or 4 toxicities. The most common adverse events included fatigue/asthenia, nausea, vomiting, gastrointestinal symptoms, and rash. Recently, phase II/III clinical trials of HuMV833 have begun in Europe.
39.2.3 VEGF-Trap
VEGF-Trap is a novel, high-affinity molecule to VEGF molecule, generated as a fusion molecule of the VEGF receptor extracellular domain and the Fc portion of immunoglobulin G1. Data from phase I studies showed the clinical feasibility of these agents, which are currently being investigated in phase II/III trials [35, 36]. In particular, VEGF-Trap R1R2 is a derivative of the soluble form of VEGFR-1, which irreversibly binds to VEGF. This VEGF blocker is a chimeric fusion molecule composed of the second immunoglobulin domain of VEGFR-1 and combined with the third immunoglobulin domain of VEGFR-2 [37]. VEGF-Trap R1R2 was engineered to minimize interactions with the extracellular matrix, maintaining its potent affinity for VEGFR-2. Preclinical studies showed tumor growth inhibition in various models [38, 39]. In a phase I study, VEGF-Trap administered to patients with solid tumors did not induce antibody response with evidence of biological activity [40]. At present, a phase I clinical is investigating the side effects of VEGF-Trap R1R2 in patients with relapsed or refractory advanced solid tumors or non-Hodgkin’s lymphoma.
39.3 Anti-VEGF Receptors Targeted Therapies
39.3.1 IMC-1C11
Among VEGF family receptor, the VEGFR 2/kinase-insert-domain-containing receptor (KDR) appear to be significantly upregulated during tumorigenesis. IMC-1C11, a chimeric monoclonal anti-KDR antibody, blocks the binding of the ligand to the receptor and subsequently inhibits downstream events such as VEGFR and MAPK activation, inhibiting VEGFR-induced endothelial cell proliferation [41]. This agent is presently being examined in a phase I trial in patients with liver metastases from CRC carcinoma. IMC-1C11 appears to be safe and well tolerated, although 7 out of 14 patients experienced detectable levels of antibodies against IMC-1C11 [42]. A fully human anti-VEGFR-2 has been produced as a second-generation agent to be less immunogenic for chronic administration as monotherapy or in combination with chemotherapy or radiotherapy [43].
39.3.2 Sunitinib Malate
Sunitinib is an oral inhibitor of VEGFR-1, VEGFR-2, stem-cell factor receptor (c-KIT), PDGFRα, PDGFRβ, and fetal liver tyrosine kinase receptor 3 (FLT3) [44–46]. A phase I study investigating the biological activity of sunitinib in patients with AML showed that the phosphorylation of FLT-3 was inhibited in 50 % of patients with wild-type FLT-3 and in 100 % of patients with mutated FLT-3. The majority of gastrointestinal stromal tumors (GISTs) harbor mutations in the receptor tyrosine kinase KIT or PDGFR-A and are responsive to imatinib. Unfortunately, tumors develop resistance due to amino acid mutations in the kinase domain of the targeted receptor, thus preventing or weakening the interaction with the inhibitor. In vitro studies have demonstrated that sunitinib potently inhibited various imatinib-resistant KIT variants [47, 48]. In phase I studies sunitinib showed a manageable toxicity and the most common side effects reported were fatigue, hypertension, sore mouth, skin, gastrointestinal and hematological toxicity [49, 50]. Among side effects, an increased incidence of hypothyroidism was reported, related to a reduced drug-induced vascularity of thyroid gland [51]. Sunitinib demonstrated activity in patients affected by NSCLC, neuroendocrine and other tumor types, although most data are available for patients affected by GIST and RCC [52, 53].
The therapeutic advantages of sunitinib were examined as second-line therapy in patients with RCC. RCC is one of the most resistant tumor types, and, cytokines were the only moderately active agents. Two phase II clinical trials evaluated the activity of sunitinib (50 mg daily administered for 4 weeks followed by 2 weeks off) in patients with RCC after failure of previous immunotherapy [54, 55]. In the first study of Motzer et al. (n = 63) 40 % of partial responses were reported and TTP was 8.7 months. In addition, 27 % of patients demonstrated stable disease lasting more than 3 months. The most common reported adverse event was fatigue (grade 3 in 11 % of patients). Therapeutic activity was confirmed by a further open-label study single-arm clinical trial as second-line treatment of mRCC that had progressed despite previous cytokine therapy. A partial response was observed in 36 of 106 patients (34 %) and the median progression-free survival was 8.3 months. Recently, Motzer et al. compared IFN-α to sunitinib as first line therapy in metastatic RCC in a phase III randomized clinical trial. Sutininib showed a statistically significant higher response rate (31 versus 6 %) and PFS (11 versus 5 months) as compared to IFN-α (HR 0.42; p < 0.001) [9].
This agent was also investigated in a phase III trial in 312 patients affected by GISTs who progressive after imatinib therapy. The trial was unblinded because a planned interim analysis showed significant better outcome in the sunitinib arm [56]. Median time to tumor progression was significantly longer in the sunitinib group compared to placebo (27.3 versus 6.4 weeks, respectively; HR: 0.33; p < 0.0001). The confirmed objective response rate was 7 % in the sunitinib group versus 0 % in the placebo group (p = 0.006). The survival benefit could be underestimated as a result of the cross-over of patients receiving placebo.
Clinical activity of sunitinib is being investigated in NSCLC and preliminary data have shown that stable disease and partial response have been achieved [52]. Adverse events were generally mild-to-moderate and included fatigue, diarrhea and nausea. On the basis of these clinical results, in January 2006, sunitinib was approved by FDA for the treatment of advanced RCC refractory to cytokine therapy and for imatinib-resistant or imatinib-intolerant GIST.
39.3.3 Sorafenib
Sorafenib is an oral inhibitor targeting multiple kinases including VEGFR-2, VEGFR-3, PDGFR, FLT-3, c-KIT, c-Raf1, and B-Raf [57]. In a phase I study sorafenib showed activity in various tumor types and a manageable toxicity profile, both in monotherapy and in association with chemotherapy [58, 59]. Dose limiting toxicity was associated with diarrhea, fatigue, hypertension, skin rash, and hematological toxicity. The dose of 400 mg twice daily was recommended for phase II evaluation, showing interesting antitumoral activity in patients with various types of solid tumors, especially renal cell carcinoma (RCC) [60–63]. Data from a large phase II randomized discontinuation trial of sorafenib in patients with mRCC demonstrated significant disease-stabilizing activity in the arm receiving the active drug compared with placebo group. During the run-in period, 73 out of 202 patients had tumor shrinkage of >25 % and median overall PFS was 29 weeks for the overall population [64]. The most common drug-related adverse effects included rash, hand–foot syndrome, and fatigue. Grade 3/4 drug-related events occurred in 47 % of patients, with the most common being hypertension (24 %), hand–foot syndrome (13 %), and fatigue (5 %) No patient died from sorafenib-induced toxicity. A subsequent phase III study including 769 patients with advanced or metastatic cytokine-refractory RCC, demonstrated antitumoral activity of sorafenib in this population and significant clinical benefit [65]. Median PFS was indeed 24 weeks in the sorafenib arm versus 12 weeks in the placebo group (HR = 0.44; p < 0.00001). Common side effects were diarrhea (33 %), rash (34 %), hand–foot skin reactions (27 %), fatigue (26 %), and hypertension (11 %). Updated results recently reported demonstrated a survival advantages as compared to placebo (median OS of 19.3 versus 15.9 months, respectively. HR = 0.77; p = 0.015) [66]. Based of these results sorafenib was approved by FDA for the treatment of advanced or metastatic RCC refractory to cytokine therapy. Feasibility of combination therapy with Interferon-α (IFNα) is under evaluation in phase II trials, as well as direct comparison between these two agents in patients with RCC [67–69]. Additional investigations are underway to better define role of sorafenib in combination with other targeted agents or cytokines in RCC and as adjuvant therapy following a nephrectomy. Ongoing phase III studies are evaluating the activity of sorafenib for treatment of HCC, melanoma, and NSCLC.
39.3.4 PTK-787/ZK-222584
Vatalanib (PTK787/ZK 222584) is a small molecule orally active TKI of all known VEGFRs, PDGFR tyrosine kinases and the c-kit protein tyrosine kinase. In phase I studies the most common side effects reported were fatigue, hypertension, nausea, vomiting, dizziness, and transaminases elevation [70–72]. Dose identified for further examination was 1250 mg daily. In phase II studies vatalanib showed clinical activity in several solid tumors [73–75]. Phase III trials did not show conclusive results in patients affected by mCRC. In fact CONFIRM-1 study, including 1168 patients with mCRC, demonstrated no beneficial effect of adding vatalanib (1250 mg once a day) to chemotherapy (FOLFOX-4) in first line treatment [76]. Adverse events attributable to PTK/ZK were generally reversible and similar to other antiangiogenic agents. In the phase III placebo-controlled CONFIRM-2 study, enrolling 855 patients with mCRC, adding valatinib to the same chemotherapy regimen (FOLFOX-4) in second line of treatment, demonstrated a longer PFS versus chemotherapy alone (5.5 months versus 4.1 months; HR: 0.83; p = 0.026). Response rate was similar in the two arms (18.5 % in the PTK/ZK arm versus 17.5 % in the placebo arm) and survival advantage was not reported (OS was 12.1 months in the PTK/ZK arm and 11.8 months in the placebo arm. HR: 0.94; p = 0.511) [77]. Recently, a meta-analysis of these two studies was performed, based on the preliminary results from CONFIRM trials that showed a greater clinical benefit and improvement of PFS in patients with high LDH levels. This analysis confirm that the effect of vatalanib in improvement of PFS is strong in high LDH population (HR 0.65, p < 0.001), as compared to overall population (HR 0.85, p = 0.005) [78].
A report of preliminary results from a phase II clinical trial investigating the therapeutic benefits of PTK-787/ZK-222584 as a single second-line agent for NSCLC showed that disease control was achieved in 58 % of patients [75]. Other clinical trials investigating PTK-787/ZK-222584 in metastatic neuroendocrine tumors and imatinib mesylate-resistant metastatic GIST are currently ongoing [74, 79].
Our group designed a dose finding phase I study with PTK787/ZK in combination with chemotherapy and trastuzumab, involving patients affected by HER-2/neu positive mBC who progressed after trastuzumab and anthracycline-based and/or taxane-based chemotherapy for metastatic disease. The schedule of study treatment includes vinorelbine (day 1-8-15, q 28 days, dose ranging from 25 to 30 mg/mq), weekly trastuzumab and daily PTK787/ZK (dose ranging from 500 to 1250 mg). Four dose levels are planned: the primary objective of the present study is to determine the maximum tolerated dose (MTD) and the pharmacokinetics interactions of PTK787/ZK with the cytotoxic agents. The secondary objective of the study is to evaluate the tolerability and the optimal schedule of the schedule.
39.3.5 AMG-706
AMG 706 is an oral small molecule multi-kinase inhibitor with both antiangiogenic and direct antitumor activity that selectively targets VEGF, PDGF, and Kit receptors [80]. It has demonstrated antiangiogenic and antitumor activity. Data from a phase I study presented at the 2005 American Society of Clinical Oncology (ASCO) meeting showed that AMG-706 in patients with advanced solid tumors was safe at doses up to 125 mg/day [81, 82]. Preliminary data were promising, as they indicated vascular changes and stable disease in the majority of patients. Most frequently reported adverse events were hypertension, fatigue, diarrhea, headache, and nausea. AMG-706 is currently undergoing several phase I/II clinical trials alone or in combination with chemotherapy in CRC, GIST, NSCLC and thyroid cancer. Among these studies, a phase I clinical trial evaluating the safety and clinical activity of AMG-706 in thyroid cancer showed that this agent was well tolerated and objective response was achieved in 43 % of patients [83]. AMG-706 is also being investigated in combination with panitumumab, an anti-epidermal growth factor receptor (EGFR) monoclonal antibody, in NSCLC, and preliminary data revealed that this regimen was safe and exhibited clinical activity [84].
39.3.6 ZD-6474 (Zactima, Vandetanib)
ZD6474 is a dual-kinase inhibitor that inhibits VEGFR-2, but also has moderate anti-EGFR activity and RET receptor. In phase I evaluation, ZD6474 was well tolerated at daily dose of 100–300 mg/day and adverse events commonly reported were diarrhea, rash, fatigue and asymptomatic QT-prolongation [85]. Preclinical studies have yielded data consistent with a potent inhibition of the VEGF signaling pathway, suggesting potential use in a broad range of tumors including colon, lung, prostate, breast, and ovarian cancers [86]. However, most clinical data in phase II studies are available in NSCLC. Adding of ZD6474 to carboplatin/paclitaxel in first line chemotherapy is safe and did not significantly increase treatment toxicity. In a phase II randomized study performed by Heymach et al., patients with locally advanced or metastatic NSCLC after failure of first line platinum-based chemotherapy were randomized to receive docetaxel plus ZD6474, either at dose of 100 mg 300 mg, or docetaxel alone. Median PFS was higher in patients receiving the combined therapy (19 versus 17 versus 12 weeks, respectively) [87]. Recently, a double-blind phase II randomized trial compared ZD6474 300 mg with gefitinib 250 mg in 168 advanced previously treated NSCLC patients, with the option of crossover at the time of progression. Preliminary data showed a response rate of 8 % in the ZD6474 arm and 1 % in the gefitinib arm and a statistically significant longer PFS with ZD6474 was reported (11.9 versus 8.1 weeks, respectively; p = 0.011) [88]. These positive results were not confirmed in BC: in 44 patients refractory to anthracycline/taxane, ZD6474 either at dose of 100 mg or 300 mg did not show clinical activity [89]. ZD6474 displayed also promising evidence of activity in patients with hereditary medullary thyroid carcinoma; in a phase II trial in 15 evaluable patients, three had partial response and ten stable disease [90].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree