Balanced immunosuppression is essential to ensure acceptance of a solid organ transplant and an overall successful patient outcome. The fundamental purpose of immunosuppression is to modulate the immune system’s ability to recognize the transplanted organ. However, an overly suppressed immune system increases the risk of certain infections in pediatric solid organ transplant recipients. The goal of balanced immunosuppression is to carefully walk the fine line between too little immunosuppression, which predisposes patients to organ rejection, and too much immunosuppression, which predisposes patients to opportunistic infections.
Although the focus on immunosuppression and its link to infection is warranted, there are other risk factors for infection in pediatric solid organ transplant recipients. Before transplant these may be similar between children and adults. Chronic disease alone is a key risk factor. Potential transplant recipients may undergo multiple rounds of antibiotic treatment for pneumonia, cholangitis, peritonitis, and urinary tract infection, thus increasing their chances of an antibiotic-resistant or opportunistic pathogen. Many potential recipients may also need hospitalization, thus increasing their exposure to multiple types of infections. Transplant candidates are often dependent on the use of central venous catheters, peritoneal dialysis catheters, hemodialysis catheters, ventricular assist devices or extracorporeal membrane oxygenation, all of which increase the risk of systemic invasion by various microorganisms.
Sources of infection after transplant broadly include donor-derived infections, infections acquired perioperatively, reactivation of latent infections, and other infections acquired throughout the patient’s lifetime after transplantation, when there is the added effect of immunosuppressive medications. Postoperatively, poor wound healing is common, and there may be open chests or open abdomens that increase infection risk.
There are also unique issues in pediatric solid organ transplant recipients that contribute to the overall risk of infection. Pediatric recipients are more likely to have malnutrition, which can affect normal immune responses. The actual transplant surgical procedure can involve smaller vascular structures, with higher risk of complications (hematoma, thrombosis). The pediatric solid organ transplant recipient is often naïve to numerous infections, as there is less lifetime exposure to infectious agents. Compounding this is the fact that many children cannot complete the full primary immunization series before transplant. All of these factors contribute to an underdeveloped protective immunity. The following sections review the important surgical and immunologic risk factors for infection in more detail, with a focus on pediatric considerations when appropriate.
Surgical considerations
Surgical infections in the pediatric solid organ transplant recipient are an important source of morbidity, particularly in the early period after transplantation. Surgical infections are broadly classified as either superficial or deep surgical site infections. The risk and nature of these infections differ by organ type.
Superficial surgical site infections
Superficial surgical site infections refer primarily to wound infections in the skin from incisions made during the transplant procedure. Most commonly, these are caused by gram-positive organisms that colonize the skin. Antimicrobial prophylaxis administered before skin incision has been proven to reduce the incidence of these infections and has been adopted for transplant procedures. Treatment of typical superficial surgical site infection includes antibiotic therapy with coverage of gram-positive organisms and local wound care. Local wound care may include exploration of any areas of induration and redness, which may harbor purulent drainage in the subcutaneous space. If such areas are found, treatment consists of reopening the skin and subcutaneous tissue, evacuating the subcutaneous fluid collection, sending any diagnostic samples for microbiologic cultures, and leaving the wound open to heal by secondary intention (granulation from the subcutaneous layer upward). Local wound care thereafter typically includes wet-to-dry dressing changes or the application of a negative-pressure dressing (wound vacuum-assisted closure).
Necrotizing wound infections represent a rare but severe form of wound infection that must be diagnosed and treated expeditiously, particularly in immunosuppressed individuals. These severe necrotizing infections are commonly polymicrobial, but can also be caused by group A Streptococcus and clostridial organisms. Presentation includes severe pain at the surgical site, high fevers, leukocytosis, and electrolyte abnormalities. These infections are characterized by rapid progression along soft tissue planes including fascia. Treatment requires intravenous antibiotic therapy and urgent operative debridement of involved tissues, which typically includes skin, subcutaneous tissue, and deeper fascia.
Deep surgical site infections.
Deep surgical site infections occur in body cavities that are exposed during the surgical procedure. Most commonly, these infections are related to the development of fluid collections in these compartments, which are either primarily or secondarily infected. The causes of deep surgical site infections vary by the type of surgical procedure performed and are discussed by organ type. In many cases, catheter-based drainage of these infected fluid collections combined with antimicrobial therapy allows prompt resolution, but in some cases surgical debridement and drainage is required.
Heart transplantation.
The most common deep space infection after heart transplantation is mediastinitis, which is characterized by a deep infection of the sternum. The incidence after heart transplantation is 2.5% to 7.5%, and risk factors include younger age (<1 year), epicardial pacing wires, and red blood cell transfusion. , Mediastinitis is typically a monomicrobial infection, with the most common etiology being both methicillin-sensitive Staphylococcus aureus and methicillin-resistant S. aureus . Treatment generally requires operative debridement of infected tissues, complex chest closure incorporating soft tissue flaps, and prolonged antimicrobial therapy.
Lung transplantation.
After lung transplantation, deep space surgical infections most commonly occur in the pleural space. Fluid and hematoma can accumulate in the pleural space and become secondarily infected, developing into an empyema if the infection progresses. Infected pleural fluid is typically managed with chest tube drainage, but if an empyema develops, surgical debridement and drainage are warranted. The incidence of empyema is approximately 3% to 5% in the lung transplant population and is associated with a significant increase in morbidity and mortality. ,
Kidney transplantation.
Deep space infection after kidney transplantation arises from infected fluid collections in the surgical bed. Kidney grafts can be implanted in either an intraperitoneal or retroperitoneal location, depending on the size of the recipient. In younger/smaller recipients, the graft is typically placed in an intraperitoneal location, using the distal aorta and inferior vena cava as sites for vascular inflow and outflow, respectively. In this setting, fluid collections that arise thereafter are located in the peritoneal cavity. Fluid collections may consist of hematoma, lymphatic fluid, or, less commonly, urine from a urine leak between the transplanted ureter and recipient bladder. Most common among these are hematomas, which can serve as a rich source of nutrient media for microorganisms.
In older/larger pediatric recipients, the kidney graft is usually placed in a retroperitoneal position, using the external iliac artery and vein for inflow and outflow, respectively. The retroperitoneal space is a more confined, limited space and is thus usually easier to manage if fluid collections develop. The same types of fluid collections (hematoma, lymphocele, and urinoma) can arise in this space and are typically well managed with percutaneous catheter drainage.
Liver transplantation.
Deep space infections after liver transplantation are common and can arise from multiple sources. The formation of a hematoma in the peritoneal cavity is very common after liver transplantation, owing to the coagulopathy that is common in both the pretransplant and early posttransplant period.
Biliary leakage is a primary source of infected fluid collections after liver transplantation. Owing to the relative scarcity of appropriately sized pediatric donors, many pediatric patients receive partial liver grafts consisting of a portion of an adult donor liver, either from a living or deceased donor. The most commonly used partial graft consists of the left-lateral section of an adult donor liver. The biliary drainage from this graft is via the left hepatic duct. Bile leaks can occur from the biliary anastomosis between the graft and a roux-en-Y limb of jejunum. More commonly, bile leaks arise from the cut surface of the liver, where the left-lateral section is divided from the remainder of the donor liver. Fortunately, most of these “cut surface” bile leaks are self-limited and well managed with surgical drains left at the time of transplant.
Multivisceral and intestinal transplantation.
Infections in multivisceral and intestinal transplantation are common owing to the intensive induction immunosuppression administered and the exposure to enteric organisms related to bowel anastomosis. A multivisceral transplant typically consists of the donor liver, pancreas, and small intestine, retrieved from the donor as a single unit (en bloc). The vascular inflow for the graft is provided by an aortic conduit arising from the recipient infrarenal aorta, and the vascular outflow for the graft is via the inferior vena cava. In an isolated intestinal graft, the donor intestine is supplied by the superior mesenteric artery and the vascular outflow by the superior mesenteric vein, which are anastomosed to the aorta and inferior vena cava of the recipient, respectively.
Deep space infections after multivisceral or intestinal transplants may arise from enteric contamination or leakage at the sites of bowel anastomosis, most commonly involving gram-negative and anaerobic organisms. In general, two separate enteric anastomoses are required: one proximal and one distal. The proximal enteric anastomosis is usually constructed between the recipient stomach/proximal intestine and the graft jejunum. The distal enteric anastomosis is constructed between the graft ileum (or colon, if it is included) and the recipient colon. A diverting ileostomy is typically created to allow endoscopic access for the protocol biopsies necessary to monitor the intestinal graft for rejection.
The other primary sources of deep space infection after multivisceral or intestinal transplantation are infected hematomas that arise in the postoperative setting, similar to the other solid organ transplants discussed previously.
Immunologic overview
Components of the immune system
There is a complex interplay within the diverse components of the immune system that helps protect hosts from infectious threats and foreign substances. , The first main component consists of the members of the innate immune system: neutrophils, macrophages, dendritic cells, natural killer cells, complement, and various signals such as cytokines and Toll-like receptors. The innate immune system provides constant surveillance against external pathogens. The second component consists of the acquired, or adaptive, immune system, including T cells and B cells, which help the immune system fine-tune the elimination of specific threats, and contribute to memory and tolerance. The acquired immune system helps regulate the overall immune response. The focus of this section is on alloactivation of the acquired immune system, specifically T and B cells, and related processes. The immune system is extremely intricate, and other immune mechanisms fall outside the scope of this chapter.
T cells are activated through a complex pathway of signals ( Fig. 1.1 ), and more than one signal is required for full activation. The major histocompatibility complex (MHC) on the antigen-presenting cell (APC) brings an antigen that binds to the T-cell receptor, known as signal 1. Additionally, a costimulatory signal, between B7 ligands (B7-1, or CD80; and B7-2, or CD86) on the APC and CD28 on the T-cell represents signal 2. Lastly, an interaction between the cytokine IL-2, and its respective receptor on the T cell is represented by signal 3. The IL-2 receptor is made of three subunits, including alpha (CD25), beta (CD122), and gamma chains.
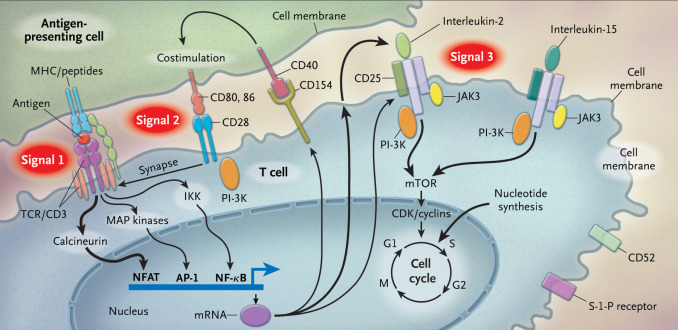
The normal process by which the host learns to recognize self from nonself includes the use of the MHC. There are two specific types of MHC complexes: class I and class II. Class I MHC is expressed by all nucleated cells and is composed of a polymorphic alpha chain, as defined by human leukocyte antigen (HLA) alleles and a highly conserved monomorphic beta-2 microglobulin chain. Nucleated cells constantly have turnover of their proteins, and the proteasome creates peptides, some of which bind to the MHC complex and are translocated across the cell membrane. The extracellular peptide–MHC is then shown to regulatory CD8 T cells, which are normally able to differentiate peptides that bear the intrinsic signature of the host, versus peptides that would indicate a foreign invader, such as a virus, or a malignant cell. Abnormal cells are then targeted for destruction. HLA alleles associated with the MHC class I complex include A, B, and C. In theory, the rise of polymorphisms in the HLA alleles helps with the immune response to a variety of infections and contributes to fitness on an individual and population level.
The class II MHC complex is present only on APCs, macrophages, dendritic cells, and B cells. The class II MHC complex is bound to extracellular protein and is presented to CD4 T cells, which help potentiate the response to foreign invaders. HLA alleles most commonly associated with the MHC class II complex include DR, DQ, and DP.
Matching based on HLA alleles has been one of the primary strategies to ensure optimal clinical outcomes. Although a perfect match may not always be feasible because of the limited number of organs available or the shortened time frame for transplant, HLA mismatch can lead to increased risk of rejection and increased use of immunosuppressive drug regimens, which ultimately lead to increased risk for infection.
Other components of the immune system are worth mentioning here as they represent targets of current immunosuppressive therapies. Regulatory T cells are important in suppressing effector T-cell function through changing the cytokine makeup, competing for the same costimulatory signals, and directing cell-to-cell signals. Cultivating the work of regulatory T cells is necessary in reaching tolerance of the transplanted organ. B cells are also pivotal in their role in both fighting infection and other foreign agents through the secretion of antibodies and facilitation of opsonization. B cells undergo different types of differentiation; a key example is plasma cells that help produce the various immunoglobulin (antibody) types. Immunoglobulins bind to specific foreign antigens and help facilitate phagocytosis and the creation of immune complexes that neutralize pathogens and activate complement. B cells can also function as APCs, in regulatory roles, and as memory cells. They contribute to the development of rejection and are therefore often targets of immunosuppressive regimens.
Lastly, the role of complement cannot be underestimated. The classic complement pathway is activated when C1q binds to the Fc portion of IgM or IgG, either in an antibody–antigen complex or on the surface of cells. Other pathways that lead to activation of complement include when the serum protein lectin binds to mannose, present on bacteria or viral-infected cells; and when complement spontaneously binds to cells recognized as foreign. The downstream target is the generation of C3b, which helps facilitate both opsonization (phagocytosis) and the creation of the terminal complement complex, which effectively punches holes in the cell membranes of pathogens and foreign cells.
Evolution of the immune system over time.
The immune system of neonates and infants in the first year of life is not well developed. The fetal innate and acquired immune system is regulated in utero to better tolerate maternal antigens that may cross the placenta. During the first year of life, although T cells are present, the response skews toward tolerance as the T cells begin to recognize self versus nonself. Although maternal antibodies do provide some immune protection for infants through the first 12 months of life, the weak response of the immune system to external threats leaves neonates and infants at high risk of serious infections. For those in this age group who receive an organ transplant, several considerations have been explored. The benefits of the immature neonatal and infant immune system have led to different strategies. ABO-incompatible liver and heart transplants are now being widely performed in young infants, with comparable results to ABO-matched transplants. Many centers consider lighter immunosuppressive regimens in infants and young children. However, given that immunosuppression is still necessary, when infection occurs in younger patients, it can take longer for acquired immunity mechanisms to recognize foreign invaders and clinical symptoms may be more severe and take longer to resolve. There is also a theoretical risk that the immune system may recognize foreign threats as self during this period, leading to mistaken tolerance.
During the transition from infancy to adulthood, children are constantly bombarded by foreign antigens through inhalation, ingestion, and inoculation. This, in turn, strengthens both innate and acquired immunity. Furthermore, the administration of routine childhood vaccination helps generate robust responses to future potential threats. Children are also exposed to the sharing of diverse antigens in different environments, including day care centers, schools, and at home. During this period children may acquire herpesviruses, such as herpes simplex virus (HSV), cytomegalovirus (CMV), and Epstein-Barr virus (EBV), which stay latent in the host. The adolescent immune system is similar to that of adults and environmental factors continue to play a role in the evolving nature of immune responses in this population.
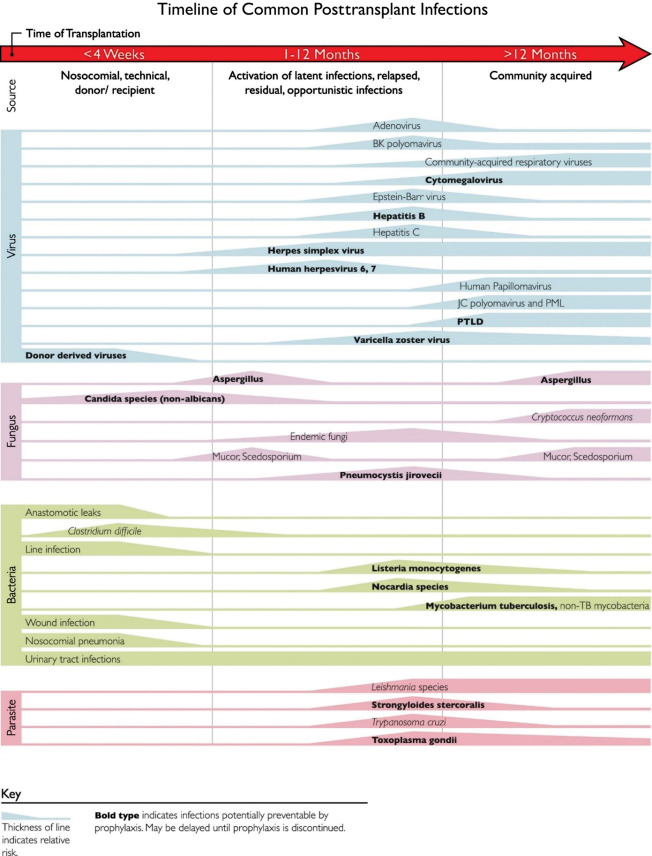
Fig. 1.2 outlines the common time line of posttransplant infections. Note that donor-derived, typical bacterial and candidal infections tend to occur earlier (at <1 month after transplant), whereas most viral and other fungal infections tend to occur later (1 to 12 months after transplant). Patient-specific and regional epidemiologic risk factors are important considerations in the evaluation of every patient. To combat these different threats, most centers have established regimens of antimicrobial prophylaxis against fungal and viral pathogens stratified by specific age group, type of organ transplant, and donor/recipient serostatus.
Rejection
One of the most common complications after solid organ transplant is organ rejection. There are different time frames of rejection, including hyperacute, acute, and chronic rejection. There are also many different types of organ rejection based on the arms of the immune system: cell-mediated and humoral. Both cell-mediated and humoral rejection can occur exclusively or simultaneously.
Hyperacute rejection
Hyperacute rejection occurs in vascularized grafts. The mechanism occurs through the action of antidonor antibodies that are already present in the recipient, leading to thrombosis in blood vessels and graft necrosis. These antibodies can occur in pediatric patients if they have been exposed to blood products or have undergone a previous transplant. Hyperacute rejection happens within minutes to hours after the transplant. Current testing methods for these antibodies include conducting flow, complement-dependent cytotoxicity, and virtual crossmatching before transplant. In these assays, recipient serum is incubated with donor lymphocytes that carry different HLA antigens, and a positive reaction would indicate that there are donor-specific antibodies. Implementation of these screenings before transplant has led to countermeasures that have significantly decreased the incidence of hyperacute rejection.
Acute rejection
Acute rejection occurs between 1 week and several months after transplant. Traditionally, this rejection has been separated into two categories: acute cellular rejection and acute humoral rejection. There are cases when one type of rejection is more dominant, but both acute cellular and acute humoral rejection can occur at the same time.
Acute cellular or cell-mediated rejection occurs when recipient T cells recognize donor tissue cells as foreign. As CD8 + cytotoxic T cells mature in the host, they begin to recognize foreign antigens in the graft through the class I MHC molecule. The cytotoxic T cells then release perforins and granzyme, which induces apoptosis. Tumor necrosis factor alpha is also secreted by the cytotoxic cells, which then leads to an inflammatory cascade resulting in both upregulated and larger numbers of immune cells at the site of the foreign antigens, leading to injury of the transplanted organ.
Acute humoral rejection is becoming a significant cause of allograft rejection. Some studies have shown that it accounts for 15% to 20% of all rejection during the first year after transplant, especially in sensitized patients. In humoral rejection, the role of B cells is important as they produce donor-specific antibodies and other cytokines, which lead to graft injury. Antibodies can be made to HLA class antigens, minor histocompatibility antigens, ABO antigens, and other non-HLA antigens. The complement system is activated and assists in creating further injury, as demonstrated by positive C4d staining in tissue specimens from affected organs.
The Banff criteria were created in 1991 to help diagnose and grade rejection for kidney transplants. For acute cell-mediated rejection, there is an emphasis on pathologic features, including interstitial inflammation, tubulitis, arteritis, and glomerulonephritis. The standardized criteria for diagnosing humoral rejection have focused on morphologic and immunohistochemical staining of tissue, as well as on serologic evidence of donor-specific antibodies. Subsequent revisions of the Banff criteria have incorporated molecular diagnostics into the diagnosis of antibody-mediated rejection. There are similar guidelines for the diagnosis of T-cell rejection in liver, heart, and lung transplant patients. However, antibody-mediated rejection and intragraft markers vary according to organ type.
The main approach to the treatment of acute cellular rejection consists of high-dose corticosteroids. If the rejection proves refractory to corticosteroids, biologic agents such as anti-thymocyte globulin (ATG) or alemtuzumab (Campath) are used. Humoral or antibody-mediated rejection is difficult to treat. Plasmapheresis, in combination with intravenous immunoglobulin (IVIG), is the first-line treatment and can be followed by other agents, including rituximab, bortezomib, and complement inhibitors such as eculizumab.
Chronic rejection
Chronic rejection is now one of the leading causes of graft rejection and failure; hyperacute rejection is becoming rarer because of the successful screening of donor-specific antibodies and more sophisticated immunosuppression regimens that have decreased the incidence of acute cellular rejection. A total of 20% of solid organ transplant recipients have graft loss by 5 years, and 50% lose their graft by 10 years after transplant as the result of chronic antibody-mediated rejection. Outcomes have improved following the more advanced treatment of acute rejection, but outcomes of chronic rejection have not changed through out the years. This affects pediatric solid organ transplant recipients as these patients have ongoing morbidities, infections, and the adverse effects of immunosuppressive medications. In addition, many pediatric transplant recipients proceed with another transplant as graft dysfunction progresses, which further increases the risk of infection.
The mechanisms of chronic rejection can include either cellular, humoral, or both processes, but is most commonly antibody-mediated. Different rounds of inflammation stimulate the expansion of memory B and T cells, which then begin to develop de novo donor-specific antibodies. Diagnostic descriptions of chronic rejection vary by organ: chronic allograft nephropathy or interstitial fibrosis/tubular atrophy in kidney transplants, cardiac allograft vasculopathy in heart transplants, bronchiolitis obliterans in lung transplants, and obliterative arteriopathy or interstitial fibrosis in liver transplants. It is thought that risk factors for chronic rejection include young age at the time of transplant, frequent infections, trauma, prior episodes of acute rejection, and medication nonadherence. Complement is often not involved in this process, and C4d staining is negative in the tissue. The long, indolent course of chronic rejection makes timely diagnosis of chronic rejection difficult. Many centers continue to screen transplant patients for donor-specific antibodies, but there is no universally accepted approach. More importantly, better therapies and further research are needed for chronic rejection as it remains difficult to treat.
Current outcomes
There is a fine balance between immunosuppression and infection in pediatric solid organ transplant patients. Overall, graft survival has increased over time as more immunosuppressive agents are now available and regimens are being optimized. There is some variation in clinical outcomes across the different types of organ transplants given distinct practices in immunosuppression. Nevertheless, infections remain a common complication in all pediatric solid organ transplants.
In pediatric kidney transplants, 10-year rates of patient and graft survival have reached 90.5% and 60.2%, respectively. There has been a significant improvement in early outcomes but limited improvement in long-term graft survival. Rates of acute rejection have fallen to 23%, but rates of infection remain at 39.6% within the first 2 years after kidney transplant. Patient age younger than 18 years was associated with a higher risk of infection in another study comparing all kidney transplant recipients.
In pediatric liver transplant recipients, using the latest data from the Studies of Pediatric Liver Transplantation (SPLIT) national consortium, patient survival rates at 3, 12, 24, and 36 months were 90.9%, 86.9%, 84.2% and 83.8%, respectively, whereas graft survival rates at 3, 12, 24, and 36 months were 85.5%, 80.2%, 76.0%, and 75.3%, respectively. Rates of rejection at 3, 12, 24, and 36 months were 44.8%, 52.9%, 59.1%, and 60.3%, respectively. In this population, infection accounted for 28.4% of deaths and was a contributing factor in 39% of deaths. Bacterial and fungal infections (not further specified) were the major infections causing death in this study.
Data from the International Society for Heart and Lung Transplantation showed that pediatric heart transplant recipients had a median survival of 22.3 years if they received a transplant in the first year of life, 18.4 years for recipients between 1 and 5 years, 14.4 years for recipients between 6 and 10 years, and 13.1 years for recipients between 11 and 17 years. Rejection during the first year after heart transplant ranged from 22% to 36%, and 5-year incidence rates of rejection reached 48%. Cardiac allograft vasculopathy affected 25% to 34% of patients 10 years after transplant. Infections caused 14% of all deaths in the first year after pediatric heart transplant, with bacterial causes in the early period, transitioning to viral and fungal causes of infection later in the first year after transplant.
The majority of pediatric lung transplants are performed in children between the ages of 11 to 17 years, and combined heart–lung transplant outcomes are integrated into the lung transplant data. Overall median survival is 5.4 years for children after transplant, but if pediatric lung transplant recipients survive past the first year, overall median survival increases to 8.8 years after transplant. Rejection rates at 1 year are 29%, and more than half of patients have bronchiolitis obliterans syndrome by 5 years after transplant. Non-CMV infection was the cause of death of 15.7% of patients at 1 month and 27.6% of patients between 1 and 12 months; this makes non-CMV infection the leading cause of death between 1 and 12 months after transplant in pediatric lung recipients.
Data on pediatric intestinal transplant patients are limited, but for a population of both pediatric and adult intestinal transplants, 1-, 5-, and 10-year survival rates have been 76%, 56%, and 43%, respectively. However, the risk of infection is high in this population, and up to 90% of patients develop a bacterial infection within the first year after transplant, and viral infections such as enteritis, CMV, and EBV infection are extremely common.
Immunosuppressive medications
The next section describes the different classes of immunosuppressive medications ( Table 1.1 ). In general, the approach to immunosuppression includes an induction regimen around the time of transplant and a maintenance regimen, with the goal of preventing rejection.