ACG 2008 [6]
AGA 2011 [3]
BSG 2014 [9]
Screening endoscopy
White male, age >50 years, GERD symptoms
White male, age >50 years, GERD, hiatal hernia, obesity
GERD >5 years, white, male, age >50 years, family history of BE or EAC
Chronic GERD symptoms + 3 (50 year or older, white, male, obese). Screening threshold lower for family history of BE or EAC in 1st-degree relative
Surveillance endoscopy
No dysplasia on 2 exams
3-year interval
3–5-year interval
3-year interval
2–3 years if max segment length ≥3 cm; 3–5 years if max length <3 cm
LGD
Repeat within 6 months and then every 12 months until no dysplasia found on 2 consecutive exams
Every 6–12 months
Repeat within 6 months and then every 12 months. Consider ablation
Every 6 months
HGD surveillance
Repeat within 3 months and then either eradication or every 3-month surveillance
Every 3 months if no eradication therapy
Endoscopic ablation
Multidisciplinary team discussion. Typically endoscopic ablation
The British Society of Gastroenterology in 2014 recommended screening patients with chronic GERD symptoms plus three of the following risk factors: age 50 years or greater, Caucasian race, male gender, and obesity (Table 8.1). They suggest that the threshold to screen for BE should be lowered if there is a family history with one first degree relative to BE or EAC (grade C recommendation) [9].
While current screening efforts focus on patients with GERD symptoms, BE is known to be present in patients without GERD, and up to 57 % of patients with EAC never report typical symptoms of GERD [2, 11, 12]. Some studies have shown that the current practice of using endoscopy after 5 years of GERD symptoms may detect only a limited number of patients who are actually at risk for progression to EAC [2, 11, 12].
A case–control study of 63 patients with EAC found that laryngopharyngeal reflux symptoms, such as asthma, aspiration, and hoarseness, might be more prevalent in those with EAC than typical GERD symptoms. Chronic cough was found to be an independent risk factor for EAC, and some have suggested that it may be helpful for screening purposes in identifying people at risk [13].
The low overall incidence of high-grade dysplasia (HGD) and intramucosal carcinoma (IMC) raises cost issues for population-based screening with conventional endoscopy [2, 14–16]. Efforts are underway to identify screening methods that may be more broadly applicable. Unsedated examinations and non-endoscopic options utilizing cytology or capsule esophagoscopy are being evaluated [17, 18]. A study involving 96 patients assessing the accuracy and feasibility of unsedated exams with disposable endoscopes compared to conventional upper endoscopy found a moderate level of diagnostic agreement between the two modalities, with Kappa coefficients of 0.409 for erosive GERD and 0.617 for BE [19]. The procedure was well-tolerated and fast and considered safe. In a study of 121 patients undergoing conventional endoscopy and unsedated small-caliber endoscopy, 71 % indicated that they would prefer to have unsedated small-caliber endoscopy performed [17]. In this study, BE was found in 26 % of the population undergoing conventional endoscopy and in 30 % of those undergoing unsedated endoscopy. The level of diagnostic agreement was moderate with a kappa of 0.591. An ingestible esophageal sampling device coupled with an immunohistochemical biomarker expressed at the BE luminal surface, called trefoil factor 3, is being studied as a non-endoscopic screening modality for BE. Trefoil 3 was discovered to be a marker of intestinal metaplasia. A study with 504 patients found a sensitivity of 90 % and specificity of 93.5 % for identifying BE of 2 cm or longer when compared to conventional upper endoscopy [20].
Surveillance
The rate of progression to cancer in non-dysplastic BE was initially thought to be close to 1 % per year in small studies [21]; however, subsequent studies have suggested a rate of progression to cancer as low as 0.12 % per year [22]. The AGA estimates the likely rate of progression to cancer to be around 0.5 % per year [3]. The goal of endoscopic surveillance in BE is to detect dysplasia, especially HGD and IMC, which can be treated through endoscopic measures before progression to invasive adenocarcinoma or metastatic disease occurs. The AGA recommends using high-resolution endoscopes (>850,000 pixels) when examining BE. The availability of this technology has allowed endoscopists to better identify areas of concern within the Barrett’s epithelium and to improve biopsy targeting of suspicious lesions. After obtaining targeted biopsies, 4-quadrant biopsies are taken every 1–2 cm for patients with non-dysplastic BE and every 1 cm for patients with dysplastic BE (whether high grade or low grade). This protocol has become the standard of care though questions arise regarding the time and cost involved with the extensive sampling and subsequent interpretation. Some research has suggested that large-capacity or jumbo biopsy forceps may also increase the amount of tissue acquired and the detection of dysplasia [23]. Use of a systematic protocol for biopsies has been shown to be more effective in detecting BE and dysplasia in BE [24]. The presence of dysplasia should be confirmed by two expert pathologists [3]. Surveillance endoscopy for BE is performed based on the highest degree of dysplasia present. If no dysplasia is identified initially, a second endoscopy with protocol-based biopsies as above should be performed within 1 year. Subsequent surveillance endoscopy should be performed every 3 years for non-dysplastic BE (Table 8.1) [6].
When BE is identified, acid reflux should be controlled with a proton pump inhibitor (PPI) in order to reduce inflammation that may disrupt visual recognition of a lesion or nodule on surveillance endoscopy and theoretically to interfere with carcinogenesis [6]. Biopsies from each segment of BE should be submitted to pathology in separate containers to better focus future biopsies on areas of concern if dysplasia is discovered.
BE is classified endoscopically according to the Prague classification [25], using C for the circumferential segment and M for the maximal length of involvement. The length of circumferential Barrett’s from the gastroesophageal (GE) junction is recorded, as is the length of the maximal extent of Barrett’s extending proximally from the lower esophageal sphincter. There is good interobserver agreement in using these criteria [25], and the approach provides a clear method of communicating the extent of the Barrett’s involvement.
If low-grade dysplasia is identified, another endoscopy should be performed within 6 months to confirm the degree of dysplasia. Surveillance endoscopy should then be performed every year until no dysplasia is identified on two consecutive exams (Table 8.1) [6]. Recent data have suggested a benefit with radiofrequency ablation (RFA) for low-grade BE, and this practice is becoming more established [26]. For BE with high-grade dysplasia, guidelines have changed, and the current approach is RFA for flat BE with high-grade dysplasia. Other options including esophagectomy and continued surveillance with upper endoscopy every 3 months may be considered in some circumstances. Endoscopic mucosal resection should be performed for areas of nodularity and mucosal irregularity prior to initiating RFA [3, 6].
The British Society of Gastroenterology (BSG) has several similarities in its surveillance guidelines compared to those of the AGA and ACG (Table 8.1). They recommend surveillance every 2–3 years for non-dysplastic BE (ND-BE) if the maximum segment length is greater than or equal to 3 cm and 3–5 years if the maximum segment length is less than 3 cm. They also recommend surveillance with endoscopy every 6 months if LGD is discovered until 2 consecutive exams show ND-BE. When HGD or carcinoma is discovered, they recommend discussion with the patient and a multidisciplinary team (MDT) determination for surveillance intervals and treatment. The MDT should include an interventional endoscopist, gastrointestinal pathologist, radiologist, and surgeon. This team should consider factors such as comorbidities, nutritional status, patient preference, and staging. The BSG suggest an outpatient discussion regarding the morbidity and mortality related to the potential treatment options, long-term survival, and quality of life [9].
A recent study from the Netherlands Cancer Registry compared patients participating in a surveillance program for BE before EAC diagnosis with those not participating in such a program between 1999 and 2009 [1]. Two-year and 5-year mortality rates were lower in patients undergoing adequate surveillance (adjusted hazard ratio (HR) = 0.79, 95 % confidence interval (CI) = 0.64–0.92) when compared with patients with a prior BE diagnosis who were not participating. This study suggested that there is a mortality reduction from EAC if adequate surveillance for BE is performed.
There are many novel and advanced imaging modalities being incorporated into surveillance endoscopy, including narrowband imaging, confocal laser endomicroscopy, and optical coherence tomography. These are promising technologies which may improve targeting and detection and may change management in patients with Barrett’s esophagus. While multiple early studies suggest their utility, they are currently being studied primarily in specialty centers and academic institutions. Broader adoption may await standardized diagnostic criteria for differentiating ND-BE, LGD, and HGD [27].
Endoscopic Treatment of Gastroesophageal Reflux Disease
Gastroesophageal reflux disease (GERD) has been implicated in the development of BE, and multiple endoscopic approaches have been studied to control GERD. Some trials have been disappointing, and thus far, no single endoscopic modality has emerged as a standard. The Stretta™ procedure involves the use of radiofrequency energy delivered through a catheter equipped with a flexible balloon-basket assembly with four electrode needle sheaths [28]. Radiofrequency energy is delivered at varying levels from the lower esophageal sphincter to the gastric cardia. This procedure was approved by the FDA in 2000 [28]. Each session lasts 60 s and can be carried out under conscious sedation or deeper anesthesia as an outpatient [29]. The procedure may lead to collagen deposition at the gastroesophageal junction (GEJ) and may increase lower esophageal sphincter (LES) pressure. It is thought that the procedure also has neuromodulatory effects from selective neurolysis of vagal afferents leading to reduced transient LES relaxations. The ablation may also decrease the perception of heartburn pain due to the influence on sensory nerves as well as reduce reflux [30–32].
EndoCinch™ is a method of endoluminal gastroplication involving suture placement at the LES for reduction of symptoms. It was also approved by the FDA in 2000 [33]. Its function is to mechanically restore a barrier against reflux. Some data suggest a decrease in esophageal sensitivity to acid after placement of the sutures [29, 34, 35]. Another device used for gastroplication is the Plicator™ which creates layered full-thickness plications of the wall of the cardia endoscopically [36].
EsophyX™ is an endoluminal device which creates an esophagogastric transoral incisionless fundoplication (TIF). It creates an anterior partial fundoplication by attaching the fundus of the stomach to the anterior and left lateral wall of the distal esophagus. Patients with moderate to severe GERD or those who are partially responsive to PPIs may benefit from treatment. Contraindications include body mass index greater than 35 kg/m2, BE, esophageal varices, hiatal hernia greater than 2 cm, and major connective tissue disorders [37, 38].
Enteryx™ was a nonabsorbable ethylene-vinyl-alcohol polymer which was injected into the musculature or deep submucosa of the LES where it solidifies into a spongelike implant in order to increase the LES pressure [39–41]. However, Enteryx™ was voluntarily recalled in 2005 for serious side effects, including death in five patients.
In summary, some data suggest that radiofrequency energy produces an improvement in GERD symptoms and quality of life with negligible morbidity [42–44] and that this approach has a good safety profile and low complication rate (<0.07 % by 2006) [45]. While initially effective, some plicating approaches may be limited by suture loss over the long term [31]. Especially in the presence of a large hiatal hernia, laparoscopic Nissen fundoplication remains a very effective approach for the treatment of GERD. Other systematic reviews suggest that because of the small number of studies with limited numbers of participants and with the side effect profiles, endoluminal therapies have not been established as the routine approach for patients with GERD [46].
Diagnosis of Barrett’s Esophagus and Esophageal Adenocarcinoma
Upper Endoscopy for Tissue Diagnosis
The standard approach for the diagnosis of EAC and BE involves visually directed biopsies obtained at an upper endoscopy. For BE, the Seattle protocol involves 4-quadrant biopsies every 1–2 cm under white-light endoscopy with the goal of detecting dysplasia [47]. The BSG guidelines published in 2014 recommend a 2 cm biopsy interval protocol in addition to the sampling of any visible lesions (BSG grade B). They state that adherence to this method is variable (10–79 %), with lower adherence for longer segments. Lower adherence may contribute to less dysplasia detection [48–50].
To establish the diagnosis of BE, endoscopic and histological criteria must be met. Upper endoscopy will show displacement of the Z-line (the squamocolumnar junction) proximal to the gastroesophageal junction (GEJ), identified by the pinch of the lower esophageal sphincter. In BE, salmon-colored Barrett’s mucosa extends proximally and is distinguished from the pale, glossy appearing squamous mucosa [51]. Pathology from the BE specimen will show intestinal metaplasia with goblet cells in the mucosa. Because of the implications for management, the diagnosis of dysplasia or adenocarcinoma should be confirmed by two expert histopathologists [9]. However, even experienced gastrointestinal pathologists may disagree on a diagnosis of HGD and intramucosal adenocarcinoma [52]. Nodularity and mucosal irregularity within the Barrett’s epithelium are more likely to contain dysplasia or carcinoma and should be targeted with focal biopsy or removed with endoscopic mucosal resection (EMR). Flat and occult lesions may be easier to detect with specialized modalities such as narrowband imaging [47].
Advanced Modalities to Improve Detection
Narrowband Imaging
Narrowband imaging (NBI) is a high-resolution endoscopic technique that enhances the imaging of the fine structure of the mucosal surface without requiring the instillation of staining agents. It involves the use of selective wavelengths of light [47]. The depth of penetration of light directly correlates to its wavelength, and increased depth of light penetration leads to a similar increase in wavelength of visible light. For instance, the blue light used in NBI allows optimal superficial imaging [53], while red light has longer wavelengths and penetrates deeper. The blue light (415 nm) and green light (540 nm) of NBI are absorbed by hemoglobin and demonstrate superficial vasculature [54]. NBI may be preferred in some settings to chromoendoscopy, which involves instillation of a dye such as methylene blue to stain the mucosa in the gastrointestinal tract for enhanced visualization. The dye in chromoendoscopy requires formulation and attention to application and may not distribute evenly over the mucosa.
A meta-analysis of eight studies including 446 patients and 2194 lesions demonstrated that the sensitivity and specificity for detecting HGD with NBI with magnification were 96 % and 94 %, respectively. The sensitivity for IMC was 95 % and the specificity was 65 % [55]. A randomized crossover trial of 123 patients showed that NBI without magnification identified a higher proportion of patients with dysplasia compared to white light (30 % vs. 21 % with p = 0.0001) [56]. A similar number of patients were found to have IMC with the use of fewer biopsies (3.6 vs. 7.6 with p = 0.0001). However, interobserver agreement regarding the interpretation of NBI images of IMC and dysplasia between expert and nonexpert endoscopists may be low [57–59]. NBI does not increase cost or add any significant risk and requires a negligible amount of time and is therefore often considered a standard part of the endoscopic examination in BE.
Confocal Laser Endomicroscopy
Confocal laser endomicroscopy (CLE) uses a low-power laser to illuminate tissue and detects the reflected fluorescent light. The laser is directed at a certain depth and light is reflected back through a very thin focal plane, refocused, and passed through the confocal aperture which enhances spatial resolution. Scanning is performed in both the horizontal and vertical planes and an in vivo microscopic image of biological tissue is produced. White-light endoscopy and CLE are performed together with images displaying simultaneously. It provides gray-scale imaging of tissue microstructures at or near the level of histopathology. These images may be at 1000-fold magnification [60]. Two devices are used for CLE including an endoscope-based confocal endomicroscopy (eCLE) system (Optiscan Pty., Ltd., Notting Hill, Australia; Pentax) and a probe-based confocal endomicroscopy (pCLE) system, which are passed down through the working channel of the endoscope (Cellvizio; Mauna Kea Technologies, Paris, France) [47].
Use of the Mainz criteria, the confocal Barrett’s classification system, demonstrated a sensitivity and specificity of 98 % and 94 % for BE and 93 % and 94 % for BE-associated dysplasia, respectively, in predicting in vivo histology [61]. Strong interobserver and intra-observer agreement was reported using this classification system (kappa 0.84 and 0.89, respectively). A randomized, controlled study involving 192 patients with BE compared high-definition white-light endoscopy (HD-WLE) with random biopsies to endoscopy plus eCLE with targeted biopsies. In this study, the combination of high-definition white-light endoscopy and eCLE increased the diagnostic yield of biopsies for neoplasia (22 % versus 6 %) and significantly lowered the number of biopsies required [62]. A multicenter study of 101 patients suggested that adding pCLE to HD-WLE significantly improved the detection of neoplasia, from sensitivity and specificity values of 34.2 % and 92.7 %, respectively, with HD-WLE compared to 68.3 and 87.8 % with combined pCLE and HD-WLE (p = 0.002 and p < 0.001) [63]. Another smaller study of 68 patients in three centers did not show as promising results when assessing pCLE versus WLE. Specificity and negative predictive values were low at 12 % and 18 %, respectively [64]. While CLE offers the promise of real time histology, it requires fluorescein administration, is expensive, has a small field of view, and requires a long learning curve. Further investigation may better define the role for the technology [47].
Optical Coherence Tomography
Optical coherence tomography (OCT) is similar to ultrasound technology but uses light waves in place of sound. It creates a cross-sectional image of tissue using infrared light by penetrating up to 3 mm in depth using a catheter through a standard endoscope. OCT does not require the administration of fluorescein. The intensity of the back-scattering of light creates cross-sectional and 3-dimensional images of tissue microstructures. The images are similar to coarse black and white histopathology. OCT does not require contact with esophageal tissue and can visualize the epithelium, basement membrane and vasculature, and lamina propria. Nuclear dysplasia cannot be observed [65]. A prospective study involving 33 patients with BE demonstrated that the sensitivity and specificity of OCT for detecting dysplasia were 68 % and 82 % [66], respectively, and the diagnostic accuracy for the four endoscopists involved ranged from 56 to 98 %. Computer-aided diagnosis (CAD) algorithms can be used to increase accuracy of detection of dysplasia and metaplasia. A recent study used histology as a reference standard and developed a CAD algorithm with a sensitivity of 82 %, specificity of 74 %, and accuracy of 83 % for detecting dysplasia in BE [67]. OCT is not currently widely available [68].
A study assessing the presence of dysplasia in BE looked at 177 biopsy-correlated images to evaluate a novel dysplasia index using OCT image characteristics of IMC and HGD in BE. The sensitivity and specificity rates for diagnosing HGD/adenocarcinoma were 83 % and 75 %, respectively [69]. There was significant correlation between diagnoses of IMC/HGD by histopathology and scores for the image features including dysplasia, surface maturation, and gland architecture [69].
Endoscopic Mucosal Resection for Diagnosis
Endoscopic mucosal resection (EMR) is recommended for patients with BE with nodules, raised lesions, or mucosal irregularity. With EMR, a specialized cap is affixed to the end of an endoscope, and tissue is suctioned into the cap. A band may be deployed at the base to create a pseudopolyp of tissue. The tissue is then removed using snare electrocautery. This technique allows the removal of approximately 1 cm area of mucosa and a portion of the underlying submucosa for histological examination. More than one EMR may be performed to resect a larger area or tissue (piecemeal EMR). EMR provides a much larger tissue specimen for examination by pathologists than traditional forceps biopsy. It is more likely to detect cancer or dysplasia, and it allows pathologists to define the precise depth of invasion in early cancers for staging [51]. The technology was originally developed as a diagnostic procedure in the 1980s and has now evolved into an effective therapeutic modality as well [4].
In a retrospective analysis involving 35 patients with BE undergoing both EMR and mucosal tissue biopsy, 63 % of specimens were discordant [70]. Fifty-three percent of biopsy results were upstaged with EMR, and the most common change was an upstaging to invasive adenocarcinoma. Only 10 % of biopsy specimens were downstaged via examination of EMR specimens. Of the 13 cases of invasive adenocarcinoma discovered through EMR, 92 % were upstaged, leading to management change in 34 % of cases. Another study demonstrated that EMR changed the grade or T-stage in 48 % of patients when compared to traditional biopsies. EMR has also been employed in eliminating the affected Barrett’s segment in 94 % of cases and has been shown to reduce the need for esophagectomy (71). EMR is a critical component in the accurate staging and proper management of BE-related lesions.
EMR may also help to diagnose invasive squamous cell carcinoma. In one study, 51 patients diagnosed with high-grade intraepithelial squamous neoplasia upon biopsy after endoscopic iodine staining was evaluated with EMR for comparison of results [71]. Histological examination of EMR specimens showed that 23.5 % (12/51) had tumor invasion of the lamina propria and 7.8 % (4/51) had muscularis mucosa invasion. The other 68.6 % (35/51) had confirmed high-grade intraepithelial squamous neoplasia. Follow-up was a median of 23 months with two recurrences both needing a second EMR.
Staging
Endoscopic Ultrasound for Locoregional Staging
Endoscopic ultrasound (EUS) is the procedure of choice to establish the depth of infiltration and lymph node (LN) status and is the most accurate tool for the TNM staging of esophageal neoplasia [72]. EUS is preceded by a careful upper endoscopic examination which provides information about the location of the disease and the extent of the background Barrett’s epithelium and also may reveal features such as gastric extension and the presence of a hiatal hernia. EUS establishes the T-stage by visualizing the wall layers and defining the depth of infiltration. EUS does not visualize nuclear and cellular changes [73], and with early-stage N0 disease, an EMR may be performed for pathologic examination to establish the precise T-stage, grade, and features such as lymphovascular invasion. EUS may not be required for HGD and small intramucosal tumors before endoscopic or surgical treatment [47].
The main use of EUS in Barrett’s-related disease has been the detection of deeply invading tumors and lymph node metastases (LNM). This can allow ablative therapy in those with disease limited to the mucosa and select submucosal tumors without malignant-appearing LNs [73]. Many studies suggest that when EUS is inaccurate, it tends to overstage more often than understage, especially in superficial Barrett’s neoplasms [74, 75]. In a study involving 125 patients with esophageal carcinoma (86 % with adenocarcinoma), EUS was 80 % sensitive for determining nodal metastasis compared to 40 % for CT (p < 0.001). The diagnostic accuracy was 81 % with EUS compared to 61 % with CT (p ≤ 0.001) [76].
Miniprobe EUS utilizes a slim catheter introduced via the working channel of an endoscope to provide high-resolution radial echoendosonographic images over a shorter depth of penetration. It can be used to examine the esophageal wall even in situations of stenosis. In a study involving 143 patients with esophageal carcinoma, 112 having EAC, 78 % of patients were accurately staged and would have been assigned to the appropriate therapy group, while 11 % were overstaged and would have been overtreated, and 11 % were understaged and would have been undertreated using miniprobe EUS to differentiate locally advanced from limited cancer.
EUS is accurate in differentiating T1 and T2 lesions and is superior to CT for lymph node staging according to a prospective trial with 100 patients with early Barrett’s-related carcinoma [77]. The T-stage diagnosed with CT was T1 or less in every patient. Using EUS the T-stage was T1 in 92 % of cases and >T1 in 8 %. Significantly more LNs were found with EUS compared to CT (28 vs. 19), and the sensitivity of CT for N-staging was low compared with EUS (38 % vs. 7 %) [77]. In another study involving 48 patients, with 8 having submucosal invasion, EUS provided accurate staging in 41/48 patients (85 %) with only one patient overstaged and 6 patients understaged compared to the histological diagnosis [78].
In another study involving 33 patients with adenocarcinoma, 21 with squamous cell carcinoma, and 1 with lymphoepithelial-like carcinoma, 86 % of the 40 T1m lesions diagnosed by EUS were confirmed by pathology. Of the 33 T1sm lesions diagnosed by EUS, 66 % were confirmed as T1sm. The accuracy of EUS in evaluation of LNM was 71 % with negative predictive value of 84 %. The accuracy by histological type was 70 % for adenocarcinoma and 81 % for squamous cell carcinoma (p = NS) [79].
Early detection of ESCC is also very important as finding and treating these lesions can lead to a 5-year survival rate of more than 90 % after endoscopic or surgical management [80]. EUS is considered to be the best option for staging early ESCC. A study showed that the accuracy of EUS for staging T1a lesions (mucosal lamina propria and muscularis mucosa infiltration) and T1b (submucosal infiltration) lesions was 70.8 % (51/72) with a sensitivity of 74.3 %. Multivariate analysis suggested that the accuracy of EUS was related to the length of the lesion (p = .029) [81] (Figs. 8.1 and 8.2).
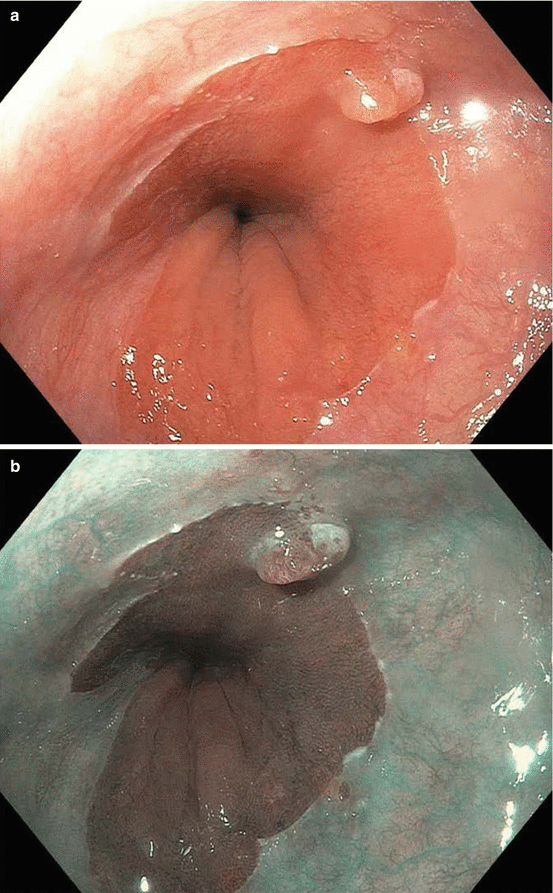
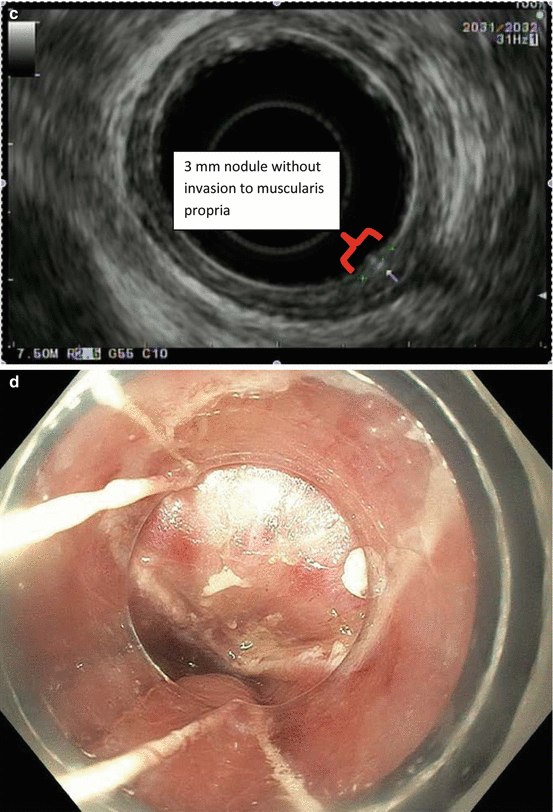
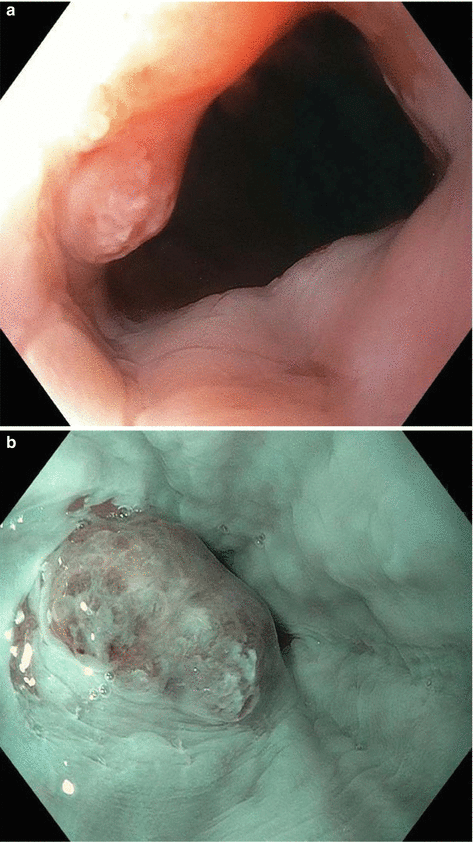
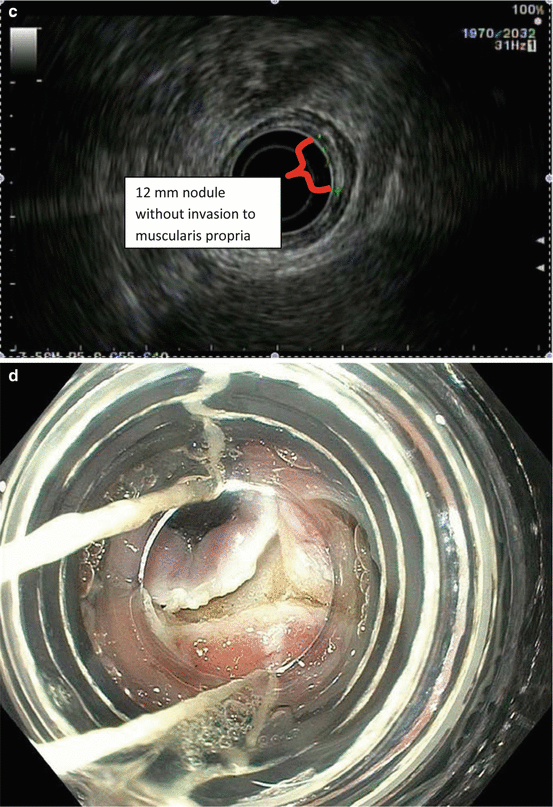


Fig. 8.1
Endoscopic staging of an intramucosal adenocarcinoma at the gastroesophageal junction. (a) 3 mm nodule at Z-line. (b) The same lesion visualized under narrowband imaging. (c) Endoscopic ultrasound showing the lesion limited to the mucosa. (d) Endoscopic mucosal resection (EMR) of the lesion. The pathology results revealed an intramucosal adenocarcinoma with 4 mm negative margins. In this case the EMR was therapeutic as well as diagnostic


Fig. 8.2
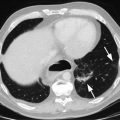
Endoscopic staging of T1b esophageal adenocarcinoma. (a) 12 mm nodular mass at Z-line. (b) The same lesion visualized under narrowband imaging. (c) Endoscopic ultrasound showing the lesion not invading the muscularis propria. For this reason, endoscopic mucosal resection was indicated for staging. (d) Endoscopic mucosal resection of the lesion
Endoscopic Treatment of Early Esophageal Cancer
Traditional therapy for early-stage esophageal cancer and BE with HGD has been esophagectomy with lymph node dissection. However esophagectomy carries significant morbidity, ranging from 20 to 50 % [82], and may have lifelong quality of life implications. In addition, the mortality from esophagectomy ranges from 2 to 10 % [82–84]. Definitive endoscopic therapy with EMR of malignancy followed by subsequent RFA of residual BE has been increasingly utilized in BE with HGD as well as early-stage esophageal cancer, defined as Tis, T1a, and T1b tumors.
Endoscopic Mucosal Resection as Therapy for Intramucosal Adenocarcinoma
As discussed above, EMR should be performed for diagnostic purposes in areas within BE with concerning features such as nodularity or mucosal irregularity. In these cases it may provide diagnostic information (precise T-stage, degree of differentiation, margins, the presence or absence of lymphovascular invasion). The precise depth of tumor invasion may further refine the treatment allocation. EMR may also be therapeutic in select cases of HGD, Tis, T1a, and certain T1b tumors, as it allows resection of the superficial layers to the submucosal layer.
The efficacy and safety of endoscopic therapy with EMR in Tis and T1a lesions have been demonstrated [85–88]. Longer term mortality outcomes for early-stage cancers have been similar between endoscopic therapy and esophagectomy [89–92]. Prospective studies have demonstrated complete oncologic eradication and low mortality with endoscopic therapy for Tis [93, 94] and T1a [93–97] lesions. The National Comprehensive Cancer Network now recommends endoscopic resection of Tis and T1a EAC followed by RFA as the preferred therapy. A recent study also demonstrated excellent outcomes with endoscopic therapy in highly selected cases with T1b adenocarcinoma limited to the superficial-most third of the submucosa (T1b sm1 lesions), though this practice remains investigational [98].
Patient selection remains the critical question when deciding between endoscopic resection and esophagectomy for early-stage tumors. Since a decision to pursue endoscopic therapy over esophagectomy implies foregoing lymph node dissection, patient selection must be aimed at identifying patients at low risk for nodal metastasis. The risk of nodal metastasis and thereby the risk of incomplete oncologic outcome can be weighed against the risk of surgical mortality in selecting a treatment modality [3, 98].
A 2012 review of 70 studies and 1874 patients with surgical pathology showed no nodal metastasis in 524 patients with HGD and 26 of 1350 patients with intramucosal carcinoma, representing a 1.93 % incidence of nodal metastasis in this group. More recently, an analysis of 715 patients with early-stage EAC undergoing esophagectomy in the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute helped to stratify patients by risk of nodal metastasis according to tumor size and degree of differentiation. There were no cases of nodal metastasis among Tis cases. Among 323 T1a cases, 6.8 % had nodal metastasis, but the incidence was 5.2 % among low-grade tumors, 2.3 % among tumors smaller than 2 cm in diameter, and 1.7 % among tumors that were both low grade and smaller than 2 cm. Among 353 T1b cases, 18.1 % had nodal metastasis, with an incidence of 8.6 % for low-grade tumors smaller than 2 cm and 3.0 % for low-grade tumors smaller than 1 cm [99].
Other than depth of invasion, size, and histological grade, lymphovascular invasion has been identified as a risk factor for nodal metastasis. Tumors with lymphovascular invasion are typically considered for esophagectomy due to the higher risk of nodal metastasis.
In a retrospective study involving 62 patients with superficial EAC, there was a local recurrence in 14 of 64 patients, 3–36 months after EMR. Larger diameter was most commonly associated with recurrence (p = 0.01) [100]. Typically, a local recurrence is managed with repeat EMR in these cases. A prospective study of EMR in patients with either early EAC or HGD in BE showed promising results for use of EMR in lower risk disease. Complete local remission was achieved in 97 % of a group of 35 patients with “low-risk” disease, including macroscopic types I, IIa, IIB, and IIc, lesion diameter up to 20 mm, mucosal lesion, histological grades G1 and G2, and/or HGD. EMR may be a less invasive option for highly selected early cancers [85].
A study of 176 patients treated for mucosal EAC (T1a) with EMR or surgery had similar cumulative mortality (17 %) with either method. Treatment modality was not a significant predictor of survival on multivariable analysis, and recurrent EAC was detected in 12 % of patients treated endoscopically. All of the recurrences were successfully retreated endoscopically without overall difference in survival [90].
In a study involving 114 patients with mucosal EAC treated surgically or endoscopically, complete remission (CR) was achieved in all patients except for one in the EMR group who died from other causes before CR could be achieved. Complications from surgery were found in 32 % of patients with 0 % major complications found in the EMR group (p < 0.001). There was a higher recurrence rate in patients who underwent EMR with one patient having local recurrence and four with metachronous neoplasia. Repeat endoscopic treatment was possible in all patients [101].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree