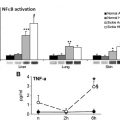
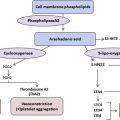
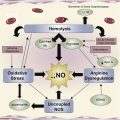
Data suggest a role for adenosine signaling in the pathogenesis of sickle cell disease (SCD). Signaling through the adenosine A 2A receptor (A 2A R) has demonstrated beneficial effects. Activation of A 2A Rs decreases inflammation with SCD by blocking activation of invariant natural killer T cells. Decreased inflammation may reduce the severity of vasoocclusive crises. Adenosine signaling through the adenosine A 2B receptor (A 2B R) may be detrimental in SCD. Whether adenosine signaling predominantly occurs through A 2A Rs or A 2B Rs may depend on differing levels of adenosine and disease state (steady state versus crisis). There may be opportunities to develop novel therapeutic approaches targeting A 2A Rs and/or A 2B Rs for patients with SCD.
Key points
- •
Activation of adenosine A 2A receptor (A 2A R) on invariant natural killer T (iNKT) cells decreases inflammation in a transgenic mouse model of sickle cell disease (SCD). The effects of regadenoson, an A 2A R agonist, are currently being examined in patients with SCD.
- •
The adenosine A 2B receptor (A 2B R) on red blood cells and corpus cavernosal cells of the penis has been implicated in the formation of sickle erythrocytes and priapism, respectively.
- •
These 2 independent lines of research examining the roles of A 2A R and A 2B R signaling in SCD may provide opportunities for new therapies.
Introduction
SCD is characterized by rigid, sickle-shaped erythrocytes, microvascular occlusion, and tissue ischemia. Sickle erythrocytes initiate the development of vasoocclusion that ultimately leads to tissue ischemia in a complex multicellular process. Tissue ischemia promotes an inflammatory response that is further amplified by ischemia reperfusion injury. Thereafter, a vicious cycle of vasoocclusion, tissue injury, and inflammation is set into motion, promoting further erythrocyte sickling. The consequences of vasoocclusion are pain, end-organ damage, and often premature death.
Therapies for preventing or treating sickle cell vasoocclusion are limited. Broadly, treatments either prevent the formation of sickle erythrocytes (eg, hydroxyurea) or interrupt the cellular interactions that follow red cell sickling and lead to vasoocclusion. Hydroxyurea stimulates fetal hemoglobin synthesis in a variable fraction of erythroblasts and thereby inhibits polymerization of sickle hemoglobin and, to date, is the only Food and Drug Administration (FDA)-approved therapy for the prevention of painful vasoocclusive crises (pVOCs). Patient and provider barriers have limited the widespread use of hydroxyurea in patients with SCD, affecting the impact of the drug. Hematopoietic stem cell transplantation is a potential cure; however, it is an option for only a few patients with SCD.
This review examines the role of adenosine signaling in SCD pathogenesis. There are opportunities to modulate adenosine pathways using therapies to prevent or treat SCD complications. As evidence has emerged about the importance of adenosine in SCD, separate lines of investigation have demonstrated protective and detrimental effects of adenosine in regard to disease severity. Recent data suggest that actions of adenosine mediated through the A 2A R decrease inflammation, largely by selectively inhibiting the activation of a subset of lymphocytes, called iNKT cells. In contrast, other studies have shown that adenosine signaling through the A 2B R may contribute to the adverse processes of erythrocyte sickling and priapism. Although much additional work is needed to fully elucidate the roles of adenosine signaling in SCD, targeting these pathways may produce novel therapeutic approaches.
Introduction
SCD is characterized by rigid, sickle-shaped erythrocytes, microvascular occlusion, and tissue ischemia. Sickle erythrocytes initiate the development of vasoocclusion that ultimately leads to tissue ischemia in a complex multicellular process. Tissue ischemia promotes an inflammatory response that is further amplified by ischemia reperfusion injury. Thereafter, a vicious cycle of vasoocclusion, tissue injury, and inflammation is set into motion, promoting further erythrocyte sickling. The consequences of vasoocclusion are pain, end-organ damage, and often premature death.
Therapies for preventing or treating sickle cell vasoocclusion are limited. Broadly, treatments either prevent the formation of sickle erythrocytes (eg, hydroxyurea) or interrupt the cellular interactions that follow red cell sickling and lead to vasoocclusion. Hydroxyurea stimulates fetal hemoglobin synthesis in a variable fraction of erythroblasts and thereby inhibits polymerization of sickle hemoglobin and, to date, is the only Food and Drug Administration (FDA)-approved therapy for the prevention of painful vasoocclusive crises (pVOCs). Patient and provider barriers have limited the widespread use of hydroxyurea in patients with SCD, affecting the impact of the drug. Hematopoietic stem cell transplantation is a potential cure; however, it is an option for only a few patients with SCD.
This review examines the role of adenosine signaling in SCD pathogenesis. There are opportunities to modulate adenosine pathways using therapies to prevent or treat SCD complications. As evidence has emerged about the importance of adenosine in SCD, separate lines of investigation have demonstrated protective and detrimental effects of adenosine in regard to disease severity. Recent data suggest that actions of adenosine mediated through the A 2A R decrease inflammation, largely by selectively inhibiting the activation of a subset of lymphocytes, called iNKT cells. In contrast, other studies have shown that adenosine signaling through the A 2B R may contribute to the adverse processes of erythrocyte sickling and priapism. Although much additional work is needed to fully elucidate the roles of adenosine signaling in SCD, targeting these pathways may produce novel therapeutic approaches.
Adenosine signaling pathway
Adenosine Physiology
Adenosine signaling protects tissues by promoting vasodilation as well as by decreasing heart rate and inflammation. During periods of cellular hypoxia or stress, adenosine is released from cells along with the adenine nucleotides, ATP, ADP, and AMP, which are converted to adenosine by ectonucleotidases. Binding of adenosine to 4 receptor subtypes, A 1 , A 2A , A 2B , and A 3 , elicits responses that are dependent on the receptor subtypes found in various tissues ( Table 1 ). Adenosine receptors are 7-transmembrane, G-coupled receptors that signal through adenylyl cyclase, affecting the production of cyclic AMP (cAMP) and calcium or the conductance of ion channels. A 1 and A 3 receptors couple to inhibitory G receptors (Gi) and decrease adenylyl cyclase activity, whereas A 2A and A 2B increase adenylyl cyclase activity by coupling to stimulatory G receptors (Gs or Go). The affinity for adenosine also differs among the receptor subtypes, affecting the concentration of adenosine necessary for activation. A 1 and A 2A are high-affinity receptors, activating at lower concentrations of adenosine (approximately 0.01 μM–1 μM). A 2B is a low-affinity receptor requiring 10- to 1000-fold higher levels of adenosine (approximately 10 μM) for activation. Downstream from adenylyl cyclase and cAMP, adenosine signaling modifies the activity of nuclear factor κB (NF-κB), JAK-STAT, and ERK pathways, regulating transcription and ultimately cellular functions. Adenosine that accumulates during cellular stress is removed by uptake into cells and converted to AMP or inosine by adenosine kinase and adenosine deaminase (ADA), respectively. In patients with SCD, tissue injury may increase levels of plasma adenosine, suggesting that adenosine pathways may influence SCD pathogenesis.
| Characteristics | Adenosine Receptor Subtypes | |||
|---|---|---|---|---|
| A 1 | A 2A | A 2B | A 3 | |
| Predominant tissue/cell expression |
|
|
|
|
| Actions |
|
|
|
|
| Affinity for adenosine | High | High | Low | High |
| Major disease associations |
|
|
|
|
Current Therapeutic Uses of Adenosine and Adenosine Derivatives
Drugs that target adenosine receptors are part of current standard practice. Adenosine, dipyridamole, and adenosine A 2A R agonists (eg, regadenoson) are used clinically to induce cardiac hyperemia during myocardial stress testing via activation of coronary artery A 2A Rs. Adenosine-mediated activation of A 1 receptors in the heart is a treatment of tachyarrhythmias. Theophylline, either alone or in complex with ethylenediame (aminophylline), nonselectively blocks A 1 , A 2A , and A 2B receptors and is used as a therapy for asthma. A limitation of these therapies is lack of selectivity for the adenosine receptor subtypes, sometimes resulting in unwanted and potentially dangerous side effects. These include hypotension and tachycardia from A 2A R activation, bradycardia or heart block from A 1 activation and bronchospasm from A 2B R activation in patients with asthma. Newer-generation adenosine agonists and antagonists have greater receptor subtype selectivity, thereby minimizing toxicities. There are emerging cellular, animal, and human data suggesting a role for the A 2A and A 2B receptors in the pathogenesis of SCD ( Table 2 ). Adenosine-based therapies are currently being examined in patients with SCD ( Clinicaltrials.gov #01788631).
| Reference | Type of Study | Transgenic SCD Model | No. SCD Patients | Agents Evaluated | Key Findings |
|---|---|---|---|---|---|
| A 2A R | |||||
| VOC | |||||
| Wallace & Linden, 2010 | Investigation of transgenic SCD mice | NY1DD | — | A 2A R agonist ATL146e |
|
| Field et al, 2013 | Phase 1 clinical study | — | 27 | A 2A R agonist regadenoson |
|
| Lin et al, 2013 | Investigation of transgenic SCD mice | NY1DD | 8 | — |
|
| A 2B R | |||||
| Red blood cell sickling | |||||
| Zhang et al, 2011 | Investigation of transgenic SCD mice, cultured human red blood cells and SCD patient blood samples | Berkley | 12 | PEG-ADA, theophylline, A 2B R antagonist MRS1754 |
|
| Priapism | |||||
| Mi et al, 2008 | Investigation of ADA-deficient and transgenic SCD mice | Berkley | — | PEG-ADA, theophylline, A 2B R antagonist MRS1706 |
|
| Wen et al, 2010 | Investigation of ADA-deficient and transgenic SCD mice | Berkley | — | PEG-ADA |
|
| Wen et al, 2010 | Investigation of ADA-deficient and transgenic SCD mice | Berkley | — | PEG-ADA, A 2B R antagonist MRS1706 |
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree