Reference
Year
Path
Arm
N
OS
MS
pCR
RT
Chemo
[33]
1988–1991
SCC 100 %
S
CRT
45
41
3y 14 %
3y 19 % NS
11 m
10.5 m NS
9.8 %
20 Gy
CDDP, 5FU
[5]
1989–1994
SCC 25 %
AC 75 %
S
CRT
50
50
3y 16 %
3y 30 %
NS
17.6 m
16.9 m
NS
28 %
45 Gy
(BID)
CDDP, 5FU, Vinblastine
[2]
1989–1995
SCC 100 %
S
CRT
139
143
3y 34 %
3y 37 %
NS
18.6 m
18.6 m
NS
21 %
37 Gy
CDDP
[34]
1994–2000
SCC 35 %
AC 63 %
S
CRT
128
128
3y 36 %
3y 31 %
NS
22.2 m
19.3 m
NS
13 %
35 Gy
CDDP, 5FU
[35]
1997–2000
SCC 25 %
AC 75 %
S
CRT
30
26
5y 16 %
5y 39 %
SS
7.8 m
19.4 m
SS
33 %
50.4 Gy
CDDP/5FU
[36]
2004–2008
SCC/AC
S
CRT
188
178
5y 34 %
5y 47 %SS
49 m
24 m
SS
29 %
41.4 Gy
Carboplatin, paclitaxel
The FFCD 9901 trial randomized 195 patients with early-stage esophageal cancer to surgery versus neoadjuvant chemoradiation therapy (45 Gy in 25 fractions with two cycles of cisplatin and fluorouracil). Seventy percent of patients enrolled had SCC. With a median follow-up of ~93 months, there were no observed differences between surgery and combined modality arms for overall survival (53 % vs 48 %) or complete resection rates (92 % vs 94 %). Local regional control was improved in patients in the combined modality arm (29 % vs 15 %). After interim analysis the trial was stopped due to futility of either treatment arm reaching superiority. Postoperative mortality was significantly increased for patients in the combined modality arm (11 % vs 3.4 %, p = 0.049) [37].
The landmark CROSS study randomized 366 patients with resectable (T1N1, T2–3 N0–1) esophageal or GEJ cancer to surgery alone or weekly carboplatin and paclitaxel with concurrent radiation therapy (41.4 Gy in 23 fractions) followed by surgery [36]. Patients were stratified based on performance status, nodal stage, histology, and treatment center. Enrolled patient characteristics included location (75 % esophagus, 22 % GEJ) and histology (75 % adenocarcinoma, 23 % SCC). Patients on the combined modality arm had improved median survival (49.4 months vs 24 months) and complete resection rate (92 % vs 69 %, p < 0.001). Pathologic complete response rate was 29 % in the neoadjuvant chemoradiation arm. Overall survival was significantly improved for the combined modality arm (HR 0.657, p = 0.003). Postoperative mortality was 4 % in both arms. An absolute 5-year survival benefit of 13 % was observed for patients in the combined modality arm (47 % vs 34 %). In addition, local recurrence rates were significantly improved for patients in the combined modality arm (34 % vs 14 %). Recurrence pattern analysis from 418 patients demonstrated a higher recurrence rate in the surgery alone versus preoperative chemoradiation arm (58 % vs 35 %). Locoregional recurrence (34 % vs 14 %, p < 0.001) and hematogenous spread (35 % vs 29 %, p = 0.025) were reduced in patients treated with preoperative chemoradiation versus surgery alone. These results suggest that the observed survival benefit is due to improved local control associated with combined modality therapy [21]. Following this work, preoperative chemoradiation has emerged as the standard of care for early-stage localized adenocarcinoma of the thoracic esophagus.
CALGB 9781 randomized patients with stage I–III SCC or adenocarcinoma of the thoracic esophagus and GEJ to surgery alone or neoadjuvant chemoradiation (50.4Gy in 28 fractions) with concurrent cisplatin and 5-fluorouracil followed by surgery. The trial enrolled 56 of a target 500 patients and closed early due to non-accrual. A pathologic complete response was observed in 40 % of patients on the combined modality arm. Intent to treat analysis with a median follow-up of 6 years showed an improvement in median overall survival (4.5 years vs. 1.8 years) and 5-year overall survival (39 % vs 16 %) for patients on the combined modality treatment arm [35].
A meta-analysis from ten trials involving 1,209 patients treated with neoadjuvant chemoradiation and surgery versus surgery alone demonstrated a 2-year overall survival benefit for the combined modality arm (33 % vs 20 %) and overall survival HR of 0.81 [31]. Meta-analysis from six randomized trials involving 764 patients treated with surgery versus neoadjuvant chemoradiation and surgery demonstrated a 3-year improved mortality rate (OR 0.53, p = 0.03) for patients on the combined modality arm. The estimated number needed to treat from this analysis was 10 [38]. Meta-analysis from nine randomized trials including 1,116 patients treated with surgery alone or combined modality therapy demonstrated a 3-year overall survival benefit (HR 0.66, p = 0.016) and improved rate of complete resection (HR 0.53, p = 0.007) [39].
An updated meta-analysis that included 12 randomized trials of neoadjuvant chemotherapy versus surgery alone included 1,854 patients and showed survival benefit for patients treated with neoadjuvant chemoradiotherapy over surgery alone [32]. Patients that received neoadjuvant chemoradiation therapy showed a significant association for improved all-cause mortality (0.78, p = 0.0001) with observed benefit for both esophageal SCC (HR 0.80, p = 0.004) and adenocarcinoma (HR 0.75, p = 0.02).
Chemoradiation Versus Trimodality Therapy
Trimodality therapy (neoadjuvant chemoradiation followed by surgery) versus definitive chemoradiation therapy has been evaluated in two randomized European trials. A French study, FF9102, randomized 259 patients with resectable (T3–4, N0–1) esophageal cancer (SCC and adenocarcinoma) to trimodality therapy versus definitive chemoradiation. All patients received neoadjuvant cisplatin and 5-fluorouracil and radiation therapy (46 Gy in 23 fractions or a split course 15 Gy in 5 fractions). Patients with a partial or complete clinical response were then randomized to surgery or additional chemoradiation therapy (cisplatin and 5-fluorouracil and 20 Gy in 10 fractions or split course 15 Gy in 5 fractions). No difference was observed in median survival for trimodality versus definitive chemoradiation (17.7 months vs 19.3 months) or 2-year overall survival (34 % vs 40 %). An improvement in local control at 2 years was observed for trimodality vs definitive chemoradiation (65 % vs 57 %) and treatment related mortality was increased in patients treated with trimodality therapy (9 % vs 1 %, p = 0.002) [40].
Stahl et al., reported on 172 patients with T3–4,N0–1 (EUS staged) SCC of the upper and mid esophagus [41]. Patients were treated with induction chemotherapy (3 cycles of 5-fluorouracil, leucovorin, etoposide, and cisplatin) followed by chemoradiation (40 Gy with concurrent cisplatin and etoposide) and then randomized to surgery or definitive chemoradiation to > 65 Gy. There was no difference in median overall survival (16 months vs 15 months) or 3-year overall survival (28 % vs 20 %) for the surgery or chemoradiation arm. Improved freedom from local progression (64 % vs 41 %) and treatment related mortality (13 % vs 4 %) were statistically significant between the surgery and chemoradiation arms.
Additional randomized trials have observed no difference in survival outcomes for patients with resectable esophageal cancer treated with surgery versus definitive chemoradiation [42, 43]. Collectively these data suggest that the addition of surgery to chemoradiation does not provide a survival benefit. Based on these observations, some argue that definitive chemoradiation is a standard of care and that surgery may serve as a salvage therapy.
The phase II RTOG 0246 study enrolled 43 patients treated with induction chemotherapy (2 cycles of 5-fluorouracil, cisplatin, and paclitaxel) followed by chemoradiation (50.4 Gy with concurrent 5-fluorouracil, cisplatin) and salvage esophagectomy for persistent or recurrent disease. With a median follow-up of 6.7 years, the estimated 5-year survival rate was 37 %. Salvage resection was attempted in 51 % of patients due to residual or recurrent disease or patient choice [44].
The role of surgery for salvage therapy following persistent disease or failure after definitive chemoradiation therapy in patients with early-stage SCC may be a reasonable management option in appropriately selected early-stage patients [45, 46].
Cumulatively these data suggest that trimodality therapy is an appropriate treatment option for patients with resectable thoracic esophageal malignancies. The underlying biological differences between SCC and adenocarcinoma likely contribute in part to observed differences in treatment outcomes from reported trials. Baseline patient characteristics and risk factors associated with SCC (tobacco and alcohol use) or adenocarcinoma (obesity, GERD, Barrett’s disease) may also contribute to observed survival outcomes and postoperative mortality risk.
Definitive Chemoradiation
RTOG 8501 randomized patients with clinical T1–3, N0–1 esophageal SCC or adenocarcinoma to radiation therapy alone (64 Gy in 32 fractions) or concurrent chemoradiation therapy (50 Gy in 25 fractions) with fluorouracil and cisplatin [4]. Patients that received concurrent chemoradiation had an improved median survival time (14 vs 9 months), improved 5-year overall survival rate (27 % vs 0 %), and improved local recurrence rate (47 % vs 65 %) [3].
Based on the promising results from RTOG 8501, Intergroup 0123 investigated the role of radiation dose escalation by randomizing patients (clinical stages T1–T4, N0–N1 SCC or adenocarcinoma) to concurrent chemoradiation therapy (fluorouracil and cisplatin) to a total dose of 64.8 Gy or 50.4 Gy. No differences were observed for median survival (13 vs 18 months), 2-year survival (31 % vs 40 %), or locoregional failure rates (56 % vs 52 %) in comparison of the high dose and low dose treatment arms. There were 11 treatment-related deaths in the high dose arm, and seven of the deaths occurred at doses less than 50.4 Gy [47]. Due to concern for treatment-related toxicity, the standard of care radiation dose has remained 50.4 Gy. The recently reported results from the multicenter phase II–III PRODIGE5/ACCORD17 trial compared definitive chemoradiotherapy consisting of 50 Gy in 25 fractions in combination with FOLFOX (fluorouracil, leucovorin, oxaliplatin) or fluorouracil and cisplatin in patients with localized esophageal cancer. With a median follow-up of 25.3 months, there were no significant differences observed in median progression-free survival (9.7 months in FOLFOX arm vs 9.4 months in fluorouracil/cisplatin arm, p = 0.64) or median overall survival (20.2 months in FOLFOX arm vs 17.5 months in fluorouracil/cisplatin arm, p = 0.70). Treatment arms were similar in regard to completion of all chemotherapy (71 % in FOLFOX arm vs 76 % in fluorouracil/cisplatin arm). While no significant differences were noted in toxicity profiles, there were more toxicity-related deaths in the fluorouracil/cisplatin arm versus the FOLFOX arm (6 vs 1, p = 0.66) [48].
Potential Toxicity and Treatment Planning
Historic radiation treatment fields for esophageal cancer have included large cranio-caudal borders due to at risk nodal volumes (ranging from supraclavicular to celiac stations) and standard superior-inferior tumor expansion (routinely 5 cm) using 3D planning techniques. A standard treatment design included AP-PA limited by spinal cord constraints followed with a boost using an off-cord multi-field arrangement. In addition to the spinal cord, organs at risk include the lungs, heart, liver, and kidneys. Dose-volume histogram constraints for lung metrics have been studied in relation to risk of radiation pneumonitis with models based on testing the normal tissue complication probability models [49]. Technical advances in radiation delivery techniques including intensity modulated radiation therapy (IMRT) enable improved conformal dose distributions with the ability to limit dose to adjacent normal tissues. Figure 12.1 depicts a representative IMRT generated plan for a patient with SCC of the thoracic esophagus. Predictive models for treatment-associated radiation-induced pneumonitis include parameters of V20, V30, and mean lung dose [50, 51]. While no randomized evidence is available for comparison of 3D versus IMRT, improved dose homogeneity and DVH parameters can be accomplished with IMRT planning [52]. Lung and cardiac dosage has been shown to be significantly reduced with IMRT compared to 3D planning (mean heart dose 22.9 vs 28.2 Gy; V30 24.8 % vs 61 %) [53]. Advances in radiation delivery techniques including planning arc therapy [54, 55], 4D planning [56], and personalized risk assessment [57] may further aid in target delineation and minimizing treatment-associated toxicities.
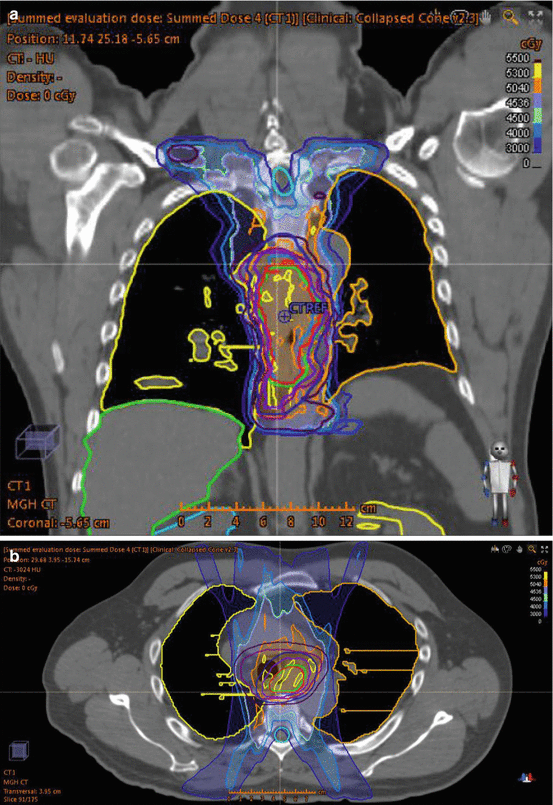
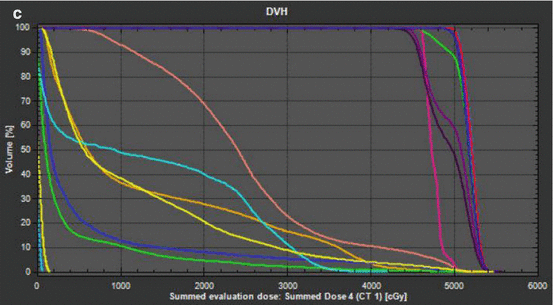


Fig. 12.1
IMRT plan for T3N0 thoracic esophagus squamous cell carcinoma. Patient planned for concurrent neoadjuvant chemoradiation therapy (carboplatin/paclitaxel) in anticipation of surgical resection. Treatment prescription for 45 Gy in 1.8 Gy fractions to CTV with 5.4 Gy in 1.8 Gy fractions boost to GTV. (a) Coronal slice of treatment plan with isodose lines. (b) Axial slice of treatment with isodose lines. (c) DVH histogram. CTV 4500 dark purple (average 4981 cGy), CTV 5040 light purple (average 5195 cGy), GTV red (average 5206 cGy), heart pink (average 2480 cGy), left lung orange (average 1277 cGy), right lung yellow (average 1136 cGy), spinal cord teal (average 1321 cGy)
Treatment Response
A complete pathologic response following neoadjuvant therapy for esophageal cancer is an important prognostic factor observed in multiple studies [5, 30, 34, 58], and post-treatment pathologic stage was highly significant for survival with combined modality therapy [59]. Estimates of 20–60 % of patients with complete response to neoadjuvant therapy have residual disease on surgical resection or pathologic review [60].
PET scans following preoperative chemoradiation have shown mixed results in regard to predicting histopathologic response and survival outcomes for patients with locally advanced esophageal cancer [61–66]. CALGB 80803 is an ongoing phase II trial assessing PET response to guide treatment in locally advanced esophageal cancer. Here, patients are randomized to FOLFOX6 (3 cycles) or carboplatin/paclitaxel (2 cycles) and treatment response is assessed by interval PET. Patients that demonstrate an imaging response (>35 % decrease in SUVmax) continue on the same chemotherapy regimen in anticipation of planned radiotherapy and surgery. Patients without favorable response are crossed over to the alternative chemotherapy in anticipation of further treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree