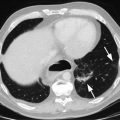
Fig. 11.1
The American Joint Committee on Cancer (AJCC) divides the esophagus into four areas. The cervical esophagus extends from the lower border of the cricoid cartilage to the thoracic inlet. The upper thoracic esophagus extends from the thoracic inlet to the tracheal bifurcation. The mid-thoracic esophagus extends from the tracheal bifurcation to a level approximately 32 cm from the incisors. The lower thoracic and abdominal esophagus extends from the mid-thoracic esophagus to the EG junction, approximately 40 cm from the incisors (Data from: Esophagus. In: AJCC Cancer Staging Manual, 6th edition, 2002)
The lymphatic drainage of the cervical esophagus includes the lower cervical chain, supraclavicular nodes, submucosal lymphatics of the proximal esophagus, and paratracheal and paraesophageal nodes in the upper mediastinum. Surgical series of neck and mediastinal dissections report metastases in 25 % and 60 % of cases, respectively [1]. Compared to hypopharynx primaries, isolated CEC has a lower tendency to involve the cervical chain but is more likely to metastasize to mediastinal nodes. The primary pattern of pathologic lymph node involvement is in the supraclavicular fossa and cervical levels II–IV, while involvement of levels I, V, and VI are rare. Unlike other esophageal sites, involvement of the upper abdominal lymph nodes (i.e., celiac/para-aortic) does not regularly occur [2].
Epidemiology
Approximately 5 % of esophageal tumors arise in the cervical segment, making it the least common site for esophageal cancer [3]. The incidence in men is twofold compared to women. Risk factors for CEC are similar to those of head and neck SCC, including tobacco smoke, alcohol consumption, and betel nut chewing. These common risk factors lead to a second malignancy rate of 30 %, primarily in other portions of the upper aerodigestive tract [4]. Certain cultural practices, such as the frequent ingestion of hot beverages, may also play a role by thermal injury [5]. Interestingly, the human papillomavirus (HPV) has been associated with a threefold increase in the risk of SCC of the esophagus [6]. Unlike head and neck cancer where HPV-related malignancy portends a favorable prognosis [7], the influence of HPV on CEC prognosis is unknown. Overall, the prognosis of CEC is poor, with a population-based registry study demonstrating a median overall survival (OS) of 14 months [8].
Presentation
Cervical esophageal cancer patients will most often present with dysphagia. The incidence of the most common symptoms at presentation include dysphagia (96 %), weight loss (61 %), neck pain (32 %), neck mass (28 %), and vocal cord paralysis (24 %) [4]. Most CECs extend to the hypopharynx or thoracic esophagus and are locally advanced at presentation [9].
Diagnosis, Workup, and Staging
The workup for CEC should include esophageal endoscopy with biopsy to confirm the diagnosis and a contrast-enhanced CT of the neck, chest, and abdomen to assess for both locoregional disease and distant metastases. PET/CT may be utilized for initial staging as it has been reported to upstage 20 % of patients as having metastatic disease compared to CT alone [10]. Endoscopic ultrasound is utilized to determine T-stage and potential nodal involvement. Accuracy in T-staging of squamous cell carcinoma is approximately 80 % [11]. The accuracy and specificity of EUS in detecting more proximal lymph node groups such as the cervical, upper paraesophageal, and supraclavicular nodes is over 90 %, while sensitivity is more variable, ranging from 30 to 60 % [12]. Bronchoscopy is recommended to rule out tracheoesophageal fistula as up to 35 % may have tracheal invasion [4]. Esophageal dilation, if feasible, may be done at the time of initial evaluation to improve dysphagia. Alternative methods for alimentation such as a jejunostomy tube should be considered prior to treatment, especially for those with obstructive lesions as well as surgically managed patients. Percutaneous endoscopic gastrostomy tubes should be avoided as they may interfere with the subsequent surgical anastomosis. A multidisciplinary team should evaluate all patients to coordinate these procedures and tailor their sequence for each individual patient.
Management
Surgery
Considering the proximity to the hypopharynx, CEC has been managed in a similar approach to hypopharyngeal cancers. Historically, surgical management was the sole treatment modality for CEC. The surgical approach for CECs typically requires removal of portions of the pharynx, larynx, and either the proximal or entire esophagus in a procedure known as pharyngo-laryngo-esophagectomy (PLE). This procedure requires a permanent tracheostomy, mediastinal dissection, and typically a bilateral radical neck dissection. Gastrointestinal continuity is accomplished with a gastric pull-up anastomosis or an interposition free jejunal graft [13].
Most published surgical series of PLE are heterogeneous including cancers of the larynx and hypopharynx. They are also limited in that few report rates of locoregional control. Historical series of surgery alone typically report high rates of operative mortality and relatively low rates of long-term survival (Table 11.1). Peracchia et al. reported one of the largest series of surgery alone including hypopharynx and CEC primaries. The overall 5-year actuarial survival was 16 % [14]. In patients treated with complete resection, those with CEC primaries had worse 5-year survival compared to hypopharynx primaries (17 vs 26 %).
Overall reports of hospital mortality rates for the procedure have ranged from 5 to 30 %, but appear to have decreased in recent years [18, 19]. Morbidity associated with PLE includes anastomotic leaks, wound infection, pneumonia, and cardiovascular complications in the perioperative period as well as the psychological consequences of a laryngectomy. Local control with surgery alone is approximately 50 % [9, 20]. The predominant pattern of failure is locoregional (80 %) [9]. These data suggest surgery alone is often inadequate when intent is curative.
Surgery with Adjuvant Therapy
Given the less than optimal results achieved with surgery alone, attempts have been made to offer multiple therapies postoperatively. One of the larger contemporary surgical series of PLE and adjuvant therapy included 209 patients, 78 of which had CEC [21]. In this series, the majority of patients received combined modality treatment (20 % neoadjuvant chemotherapy and/or radiation, 73 % postoperative radiotherapy). Hospital mortality rate was 5 % with significant complications occurring in 38 % of patients. The 5-year survival rate for CEC was 14 %. On multivariate analysis, predictors for poor outcome included cervical esophageal primary, T3–T4 stage, and lack of postoperative radiotherapy. Rates of local control either overall or with the different treatment strategies were not reported.
Wang et al. published their experience of 41 patients treated with PLE for SCC involving the pharyngoesophageal junction. Tumors were further subdivided in two groups based on their likely site of origin, hypopharynx (n = 26) or cervical esophagus (n = 15). In this study, 51 % of patients received postoperative radiotherapy, generally 3–4 weeks after surgery with a mean dose of 47.5 Gy. The overall 5-year survival was 32 %, but was only 13 % when considering cervical esophagus as the primary site [22]. On multivariate analysis, primary location in the cervical esophagus and lack of adjuvant radiotherapy were poor prognostic factors.
The most recent comprehensive report on PLE comes from an institution in Hong Kong with significant experience with the technique [18]. In this report, 62 patients underwent primary PLE, 60 % of which were R0 resections and 60 % received adjuvant radiation therapy. In-hospital mortality and 2-year OS were 7 % and 38 %, respectively. Rates of local control or the prognostic impact of adjuvant therapy were not reported.
Concurrent Chemotherapy and Radiation
Considering the morbidity and relatively high potential mortality of PLE, radiation therapy (RT) combined with chemotherapy has evolved as an alternative and often preferred modality, with the benefit of functional larynx preservation. Randomized trials primarily including thoracic or abdominal esophageal cancers have influenced the practice patterns for CEC. In a landmark trial, Radiation Therapy Oncology Group (RTOG) 85–01 randomized 129 patients with esophageal cancer to 64 Gy RT alone or 50 Gy concurrent with combination cisplatin and 5-fluorouracil (5-FU). The majority of tumors in this trial were in the upper to middle thoracic esophagus and of squamous histology. The combination of chemotherapy and radiation resulted in a significant OS improvement, with 26 % of patients in the combined modality arm alive after 5 years compared to no survivors in the radiation alone group [23]. The addition of chemotherapy also decreased the incidence of persistent disease and distant failure. This combined modality approach has since been extrapolated to most treatment platforms for CEC.
Two randomized controlled trials have investigated whether adding surgery to CRT improves outcomes for esophageal cancer patients. In the first trial, French investigators randomized 259 patients primarily with squamous histology thoracic esophageal cancers to CRT alone or CRT followed by surgery. Two-year survival was not statistically different, with 34 % alive in the CRT + surgery arm and 40 % in the CRT only arm [24]. Three-month mortality was significantly higher with the inclusion of surgery. Locoregional recurrence was reduced with trimodality therapy (34 vs 43 %). The second trial from Germany included 172 patients with squamous histology involving the upper/mid-esophagus. Patients were randomized in a similar fashion to CRT + surgery or CRT alone. Again, no significant difference in overall survival was noted although local progression was reduced with trimodality therapy. Of note, both of these trials used radiation techniques that are not considered standard for esophageal cancer including split courses, twice-daily fractionation, and/or brachytherapy. Nevertheless, the routine use of surgery after CRT did not improve survival in these patients.
Randomized data from studies of the treatment of hypopharynx cancers has also been extrapolated for the treatment of CEC. In patients with hypopharynx cancers being treated with a larynx-preserving strategy, French investigators randomized patients to induction chemotherapy followed by RT or to a concurrent CRT approach with cisplatin. The number of patients included in the trial was relatively low, limiting power to detect survival differences; however, the concurrent CRT arm had significantly higher rates of larynx preservation (92 vs 68 % at 2 years) [25]. A large meta-analysis of chemotherapy in head and neck cancer (MACH-NC) reported the benefit of the addition of chemotherapy to radiotherapy across several tumor sub-sites. In patients with hypopharynx cancer, the hazard ratio of death associated with the addition of chemotherapy was 0.88 (95 % CI: 0.80–0.96), with an absolute 5-year OS benefit of 4 % [26]. This survival benefit was primarily attributed to the use of concurrent chemotherapy with platinum-agent monotherapy or combination chemotherapy regimens.
The European Organisation for Research and Treatment of Cancer (EORTC) conducted a randomized trial in patients with predominantly hypopharynx primaries, comparing a surgical approach including total laryngectomy, partial pharyngectomy, and neck dissection followed by postoperative RT to induction chemotherapy followed by definitive RT to 70 Gy. At 10 years of follow-up, there was no statistically significant difference in OS, and more than half of survivors in the nonoperative arm retained a functional larynx [27]. These randomized data suggest the feasibility of an organ-preserving approach in the adjacent cervical esophagus.
When specifically considering CEC, relatively large retrospective institutional series have provided expected results for the use of CRT compared to PLE +/− adjuvant therapy. Investigators from Hong Kong reported their institutional experience of patients with CEC treated with PLE or CRT. From 1995 to 2008, a total of 107 patients were treated with PLE, CRT, or palliative treatment. In the upfront CRT group, patients were treated to a total dose of 60–68 Gy concurrent with cisplatin and 5-FU. Of these patients, 30 % had a clinical complete response (CR), while 50 % had downstaging. Of the entire CRT group, 24 % ultimately required salvage PLE. The median survival of patients in the PLE and CRT arms was not significantly different (20 vs 25 months, respectively) [18].
A prospective trial from Italy examined the effects of a combined modality approach for patients with SCC of the esophagus, approximately one-third of which were located in the cervical esophagus. Patients were treated with combination cisplatin (100 mg/m2 per day) on day 1 and fluorouracil (1000 mg/m2 per day) on days 1–4 for two cycles and 30 Gy RT. Patients were subsequently assessed for surgery, and those not deemed to be operative candidates were treated with additional chemotherapy and 20 Gy RT (50 Gy total). A nonoperative approach was favored for CEC, with PLE reserved as salvage treatment for recurrent or persistent disease involvement. Of the nonsurgical patients, 61 % achieved complete clinical remission [28]. The median survival in the surgical and nonsurgical groups were 12 and 22 months, respectively, with significant procedure-related mortality likely contributing to worse survival in the surgical group. Larynx preservation was achieved in 30 % of the patients with CEC in this trial.
These series suggest definitive CRT has equivalent or improved outcomes compared to a primary surgical approach. Patients with persistent or recurrent disease following CRT should be assessed for salvage surgery, although outcomes are generally poor in this population. For example, in one high-volume surgical center, in an analysis of 12 patients treated with salvage surgery between 1990 and 2005, 42 % of patients had one or more postoperative complications. While only one patient died of postoperative complications, the cause of death was recurrent cancer in 83 % with a median survival of 21 months [29]. PLE is technically more demanding in an irradiated neck and postoperative complications may be enhanced, limiting the success of this strategy.
Seeking improved outcomes by limiting the extent of surgery, an alternative approach was described by German investigators where patients underwent preoperative CRT followed by a limited resection and free jejunal graft interposition. Prior to the year 2000, patients were treated to 30 Gy in 2 Gy daily fractions with continuous infusional 5-FU. Subsequently, patients were treated to 45 Gy in 1.8 Gy daily fractions with 5-FU+/− cisplatin. In this series, a pathologic complete response was seen in 29 % of patients, with 76 % having an R0 resection [30]. Despite high complication rates, in-hospital mortality was low, and an impressive median survival of 30 months was reported. Similar to other neoadjuvant results, patients with a complete tumor response had a favorable prognosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree