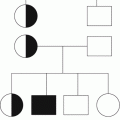
Fig. 18.1
Summary of clinical and molecular features in MPN
18.3 Diagnosis and Natural History of MPN
18.3.1 Diagnosis
The discovery of the JAK2V617F mutation has also had a major impact on the diagnostic process for these patients. In the past, these diagnoses were made by exclusion and frequently involved multiple tests to exclude other potential disorders such as an underlying malignancy in patients with isolated thrombocytosis. The current diagnostic criteria for MPN are shown in Table 18.1. It is apparent that any patients with sustained elevation of hemoglobin (i.e., a hematocrit or packed cell volume (PCV) of more than 0.51 for men and 0.48 for women), elevated platelet count (in excess of 450 × 109/L), and splenomegaly should be investigated. These disorders should also be suspected in patients presenting with thrombosis in an unusual site; for example, 60 % of patients with no clear cause for a splanchnic vein thrombosis have been reported to have a JAK2V617F-positive MPN [8]. In the context of pregnancy, it is important to consider a diagnosis of MPN in a patient with adverse pregnancy outcome, even if the blood count is normal; this is because the dilutional effect of the expanded plasma volume during the second and third trimesters may cause blood counts to completely normalize in these conditions.
Table 18.1
Diagnostic algorithm for MPNs
Careful clinical assessment to evaluate for secondary cause and additional risk factors | |
Screen for V617F JAK2 ± CMPLW515L/K | |
Positive V617F JAK2 | Negative V617F JAK2 |
PV: if V617F JAK2 and all of: | PV: if 1 + 2 and either one A or two B criteria: |
1. PCV >0.51 in Males and >0.48 in Females or raised red cell mass | 1. PCV >0.60 in Males and >0.56 Females or raised red cell mass |
2. No secondary cause of high PCV | 2. No secondary cause of high PCV and normal erythropoietin |
3. Normal/low serum erythropoietin | A Palpable splenomegaly |
A Presence of acquired cytogenetic abnormality | |
B Neutrophilia (>10 × 109/L; or >12.5 × 109/L in smokers | |
B Splenomegaly on imaging | |
B Endogenous erythroid colonies or low erythropoietin | |
B Thrombocytosis (plts >400 × 109/L) | |
ET: if V617F JAK2 and all of: | ET: if all of: |
Platelet count > upper limit normal range | Platelet count >600 × 109/L |
No other myeloid disease (includes PV, MDS, MF) | No other myeloid disease (includes CML, PV, MDS, MF) |
No reactive cause and normal iron status | |
MF: if V617F JAK2 and: | MF: if all of: |
Reticulin > grade three-fourths or presence of collagen | Reticulin > grade three-fourths or presence of collagen |
Absence of V617F JAK2 and BCR/ABL | |
And any two of: | And any two of: |
Palpable splenomegaly | Palpable splenomegaly |
Unexplained anemia | Unexplained anemia |
Teardrop red cells | Teardrop red cells |
Leucoerythroblastic film | Leucoerythroblastic film |
Systemic symptoms (night sweats, >10 % weight loss, bone pain) | Systemic symptoms (night sweats, >10 % weight loss, bone pain) |
Biopsy proven extramedullary hematopoiesis | Biopsy proven extramedullary hematopoiesis |
18.3.2 Natural History
The patient with MPN has an enhanced risk of thrombotic complications. This increased risk is thought to be due to platelet-platelet and platelet-leukocyte interaction, possibly directly related to the pathological activation of JAK2, although other as yet unclear factors are likely to contribute, for example, perturbation of the bone marrow microenvironment. Yet, not all patients will suffer from this complication, and thus risk assessment has been developed to tailor treatment strategies appropriately. It is well recognized that the risk of thrombosis latent to MPN is increased by age and history of a prior thrombotic event [9]. Interestingly, there is poor correlation between the degree of elevation of blood counts and thrombosis. However, patients with a platelet count in excess of 1,500 × 109/L are considered as a high-risk group because of their predisposition to hemorrhage (see below). Many clinicians would also include patients currently receiving antihypertensives and hypoglycemic agents as high-risk candidates. Hemorrhage, usually characterized by mucocutaneous bleeding, is less common and may be due to a reduction in the number of high molecular weight von Willebrand factor multimers, which are thought to bind to the megakaryocyte and platelet membranes. This causes an acquired von Willebrand disease (vWD), but the presence of these laboratory features may not necessarily indicate risk of future clinically significant acquired vWD. These and other factors should be considered to assess risk in the context of the current or indeed planned pregnancy; this is discussed in further detail below.
18.4 The Potential for Genetic Susceptibility to MPN
Patients diagnosed with MPN in their youth pose particular management problems over and above those faced by older patients. These include the potential for enhanced risk of long-term treatment toxicity, as well as management of fertility and pregnancy, including consideration of potential heritability of the disease. An inherited tendency to develop MPN was previously assumed to be uncommon. However, during the 1990s, studies of large kindreds with an apparent predisposition to an MPN phenotype (i.e., thrombocytosis) allowed the identification of mutations in 5′ untranslated [10] region of the thrombopoietin (TPO) gene and in cMpl, the cognate receptor for TPO [11]. Interest in a potential genetic predisposition to MPN was rekindled following the discovery of the JAK2V617F mutation in 2005, as previously described. However, this mutation has not been reported in constitutional DNA, and families have been reported in which members affected with MPN are discordant for the JAK2V617F mutation. In addition, epidemiological studies indicate that inherited factors may predispose to the development of many MPNs. The largest study assessed the risk of MPN in 24,577 first-degree relatives of 11,039 MPN patients in Sweden. The results suggest that these relatives have a significantly increased risk of developing an MPN. For example, a first-degree relative of a subject with PV has a relative risk (RR) of 5.7 (95 % confidence interval 3.5–9.1); for ET, the RR is 7.4 (3.7–14.8). For all of these cases, there was no evidence of anticipation of the age at presentation [12]. In Spring 2009, three papers appeared in the same edition of the journal Nature Genetics describing a common haplotype (single-nucleotide polymorphism) designated 46/1 conferring susceptibility to JAK2V617F-positive MPNs [13–15]. The exact mechanism of this predisposition remains uncertain, but one hypothesis suggests the “fertile ground” idea, that is, that the presence of 46/1 increases the probability of the V617F mutation occurring downstream. Despite this evidence of genetic susceptibility, routine testing of the offspring of MPN patients would not at present be recommended.
18.5 Management of MPN
Patients with MPN are generally treated according to an assessment of their risk of thrombotic or hemorrhagic potential (Fig. 18.2). Agents such as low-dose aspirin (LDA), hydroxycarbamide, anagrelide, or interferon alpha are used to reduce the risk of thrombotic events; patients with PV may require venesection and aspirin, either alone or in addition to the cytoreductive agents listed. LDA and low molecular weight heparin (LMWH) are variably used in pregnancy, which is clearly a time of transient increased thrombotic risk. However, of the cytoreductive agents, only interferon alpha would generally be recommended for the management of high-risk patients in pregnancy. Hydroxycarbamide and anagrelide both have potential to harm the fetus [16] and should ideally be stopped prior to conception (see below); for male patients wishing to father a child, both anagrelide and interferon are appropriate, but hydroxycarbamide should be stopped for 3 months prior to trying to conceive and substituted with another agent where appropriate. There is increasing interest in the utility of a pegylated interferon as a better-tolerated agent with the potential to induce complete remission at a clinical and molecular level in a cohort of patients [17]. However, the safety of this agent in pregnancy has yet to be established. JAK2 inhibitors are currently the subject of clinical trials, largely in patients with PMF, and are not yet licensed or considered standard of care. Only a very small proportion of patients with MPN will undergo bone marrow transplantation when the severity of their disease merits this high-risk approach; the management of fertility and pregnancy in this cohort of patients is beyond the scope of this text.
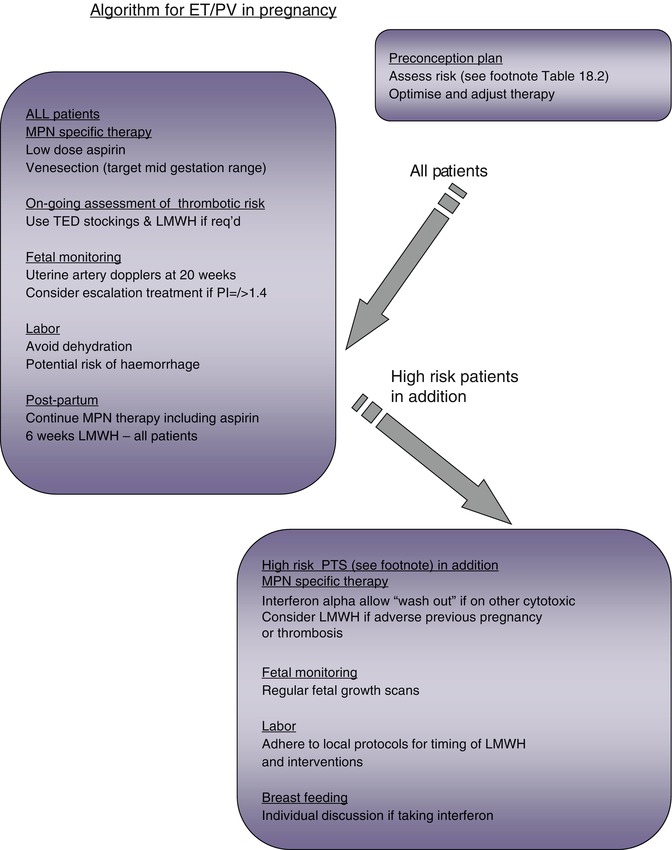

Fig. 18.2
Algorithm for MPN management in pregnancy
The development of long-term complications of ET and PV, such as transformation to acute leukemia and the more aggressive disorder myelofibrosis, appears to be dependent upon time. These complications are an inherent tendency of these conditions, possibly related to genetic instability that has been described in association with the JAK2V617F mutation. Leukemic transformation may also be related to the use of drugs such as alkylating agents. However, current therapeutic options are not thought to significantly increase the risk of leukemic transformation although there is ongoing debate with regard to the leukemogenic potential of hydroxycarbamide [9]. Whether treatment strategies can influence the risk of myelofibrotic transformation is currently the subject of debate. Pregnancy does not affect the risk of either leukemic or myelofibrotic transformation.
18.6 Pregnancy Outcomes
ET is the commonest MPN in women of child-bearing age, and a significant number of pregnancies have been described in the literature, but these data do not enable confident management guidelines to be drawn up. A number of factors have variably been used to identify high-risk pregnancies in patients with MPN, and these are shown in Table 18.2. Analysis of pregnancy outcomes in women with ET suggests that the presence of JAK2V617F may increase the risk of pregnancy loss [18]. However, the strength of this association is not sufficient to recommend adjusting management strategy on this basis. It should be noted that thrombophilia screening, unless in specific situations (e.g., a strong personal or family history of thrombotic events), does not contribute to risk assessment for pregnancy in MPNs. The current literature for pregnancy outcome in MPN is relatively sparse, and it is important to recognize that it is likely to be subject to reporting bias; the details are summarized as follows:
Table 18.2
Risk factors for complications in pregnancy
Marked sustained rise in platelet count rising to above 1,500 × 109/La |
Previous venous or arterial thrombosis in mother (whether pregnant or not) |
Previous hemorrhage attributed to ET (whether pregnant or not) |
Development of persistent notching of uterine artery Dopplers |
Previous pregnancy complication that may have been caused by ET, for example: |
Unexplained recurrent first trimester loss (three unexplained first trimester losses) |
Intrauterine growth restriction (birthweight <5th centile for gestation) |
Intrauterine death or stillbirth (with no obvious other cause, evidence of placental dysfunction, and growth restricted fetus) |
Severe preeclampsia (necessitating preterm delivery <34 weeks) or development of any such complication in the index pregnancy |
Placental abruption |
Significant ante- or postpartum hemorrhage (requiring red cell transfusion) |
ET: As ET has a second peak in incidence in women of reproductive age, the medical literature for these patients is much more abundant than for the other MPNs. A meta-analysis reported data from 461 pregnancies in women with ET whose median age was 29 years and platelet count at the outset of pregnancy 1,000 × 109/L, declining to 599 × 109/L in the second trimester. The live birth rate ranged from 50 to 70 %; first trimester loss affected 25–40 % and late pregnancy loss 10 %; the rate of placental abruption was 3.6 % and intrauterine growth restriction (IUGR) 4.5 % [19]. Maternal morbidity is rare in ET, but stroke has been reported [20].
PV: The reported outcomes of 18 pregnancies combined with a literature review of an additional 20 cases is available in the literature; outcomes were concordant with those for ET [21]. First trimester loss was most prevalent, occurring in 21 % and late pregnancy loss in 18 %; IUGR was reported in 15 % and preterm delivery in 13 %, associated with three neonatal deaths. The overall successful pregnancy rate was 50 %. Maternal morbidity was significant including one maternal death due to thrombosis and disseminated intravascular coagulation. It should be noted, however, that the literature review included cases reported in the 1960s, at which time treatment had not yet been standardized.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree