The Importance of Infection Control in Controlling Antimicrobial-Resistant Organisms
Cassandra D. Salgado
Barry M. Farr
INTRODUCTION
Infection control (IC) programs were created decades ago to control healthcare-associated infections (HAIs), including those caused by antimicrobial-resistant organisms (AROs). The success of these programs has varied from nation to nation, often dependent on resources available to devote toward effective IC programs as well as the existence of official policies and/or regulations for IC training and compliance. This chapter discusses the importance of using IC measures to prevent healthcare-associated transmission of two AROs, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococcus (VRE). Use of IC measures is important for controlling these pathogens because the measures have prevented both spread and infections (1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109). An assumption will be made that preventable infections causing serious morbidity and mortality should not be allowed to continue spreading, but should be controlled using available data to guide control efforts. MRSA and VRE are two of the most important pathogens in healthcare facilities as infections due to these organisms continue to be common and have often been associated with more prolonged illness, higher excess costs, and higher risk of death as compared with infections due to antibiotic-susceptible strains of the same species (110,111,112,113,114,115,116,117,118,119,120,121,122,123).
EPIDEMIOLOGIC DATA REGARDING THE RISK FOR COLONIZATION OR INFECTION BY ANTIBIOTIC-RESISTANT ORGANISMS
Effective prevention of any illness depends on having reliable data demonstrating modifiable risk factors. Many studies of ARO colonization and infection have identified two primary, potentially modifiable causes. The first cause appears to be clinical use of an antimicrobial. For example, before clinical use of penicillin began in the mid-1940s, resistance among clinical isolates of S. aureus was rare, but after 3 years of treating infections at the Hammersmith Hospital in London, about half of S. aureus clinical isolates had become resistant to penicillin. The relationship between the use of an antimicrobial and the development of resistance has varied for different microbe-drug pairs, but clinically important antibiotic resistance usually starts showing up about a year or two after the onset of widespread use of an antibiotic. This occurred with resistance to methicillin among clinical isolates of S. aureus; however, treating S. aureus infection with methicillin (or another antibiotic) will virtually never result in that strain of S. aureus developing resistance to methicillin de novo. Also, treating a patient with vancomycin will virtually never result in the patient’s own strain of enterococcus developing resistance to vancomycin de novo (124,125). Additionally, surveys have shown that 25% to 50% of patients in U.S. hospitals and virtually all patients in intensive care units (ICUs) receive antimicrobial therapy (126), and many studies have confirmed the importance of exposure to antibiotics as a risk factor for healthcare-associated ARO colonization or infection (127,128,129,130).
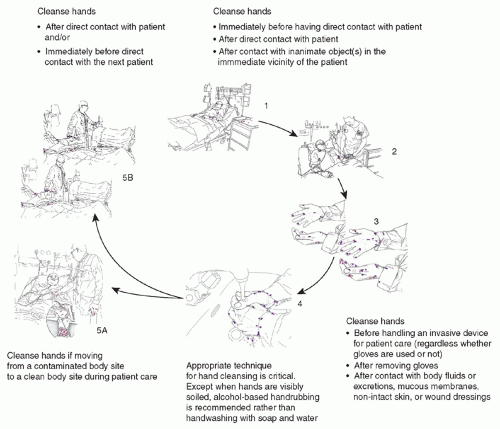
The other important cause of ARO colonization or infection has been patient-to-patient spread. The spread of lethal pathogens via contaminated hands or clothing of healthcare workers (HCWs) has been recognized for more than a century (131,132), and transmission of ARO pathogens has been documented repeatedly (12,46,93,133). Transmission among patients likely often involves transient contamination of HCWs’ hands (134,135,136,137), apparel (135,138), personal equipment (139), or medical equipment that can recontaminate the HCWs’ hands or serve as a fomite vector (90,135,140,141,142,143,144,145). Clinicians moving between patients often fail to cleanse their hands (146) and almost never disinfect their clothing or personal or medical equipment between patient contacts. A summary of the importance of transmission of organisms to patients via the contaminated HCW is demonstrated in Figure 41.1 (147).
Studies controlling for antimicrobial therapy have concluded that colonization pressure (i.e., the prevalence of colonization) and spread from other colonized patients is the most important predictor of a patient becoming colonized by MRSA or VRE (148,149,150). The contaminated environment from colonized and infected patients is also an important variable in assessing the risk of colonization pressure and transmission. For example, a recent study found that weekly colonization pressure adjusted by the degree of environmental contamination was significantly associated with MRSA acquisition in subsequent weeks (150). Similarly, proximity to nonisolated, newly colonized patients was the most important predictor of acquiring VRE in a clonal outbreak; by contrast, proximity to VRE-colonized patients in contact isolation was not a risk factor (93). Crowding and decreased nurse-to-patient ratios (44,45) make contamination of HCWs’ hands, apparel,
or equipment more likely and, thus, transmission more likely. Severe illness necessitates more touching over a longer time and usually antimicrobial therapy, each of which increases risk. In addition to transmitting MRSA or VRE from patient to patient, HCWs sometimes become colonized and transmit the organism to patients without needing to become transiently contaminated by a colonized patient (46,151,152,153,154,155,156,157,158,159). This has been mostly demonstrated for MRSA, and outbreaks in healthcare facilities have implicated HCWs in the initiation or propagation of MRSA transmission. Some describe HCWs who had active MRSA infections, such as skin infection, sinusitis, or otitis media (154,155,156), and others implicated asymptomatically colonized HCWs (157,158,159). In most of these reported outbreaks, however, transmission was halted after appropriate treatment or reassignment of the implicated HCW.
or equipment more likely and, thus, transmission more likely. Severe illness necessitates more touching over a longer time and usually antimicrobial therapy, each of which increases risk. In addition to transmitting MRSA or VRE from patient to patient, HCWs sometimes become colonized and transmit the organism to patients without needing to become transiently contaminated by a colonized patient (46,151,152,153,154,155,156,157,158,159). This has been mostly demonstrated for MRSA, and outbreaks in healthcare facilities have implicated HCWs in the initiation or propagation of MRSA transmission. Some describe HCWs who had active MRSA infections, such as skin infection, sinusitis, or otitis media (154,155,156), and others implicated asymptomatically colonized HCWs (157,158,159). In most of these reported outbreaks, however, transmission was halted after appropriate treatment or reassignment of the implicated HCW.
The high frequency of antimicrobial therapy in healthcare facilities and frequent healthcare-associated spread of microbes from person to person give AROs a selective advantage to survive, proliferate, and spread (11). This means that without effective IC measures, ARO infections tend to increase to a high prevalence within a ward/unit (44,160,161), hospital (12,19,162,163), region (133,164), and nation (165).
IMPORTANCE OF USING INFECTION CONTROL TO CURB ANTIMICROBIAL-RESISTANT ORGANISMS IN REGARD TO EFFECTIVENESS
Infection control strategies, including those to prevent transmission or infection of AROs, have recently been discussed in terms of “horizontal” measures and “vertical” measures. Horizontal measures are those that would be expected to reduce spread or infection from a variety of pathogens, and include hand hygiene, barrier precautions, environmental cleaning/disinfection, antimicrobial stewardship, and device-associated infection (DAI) prevention bundles. Vertical measures are those that are more pathogen-specific and include active surveillance testing for, and often, decolonization therapy.
DAI prevention bundles, such as those designed for central line-associated bloodstream infections (CLA-BSI) and ventilator-associated pneumonia (VAP), have been developed and implemented in many healthcare facilities (166,167,168,169,170,171,172). Sustained success of controlling HAIs has been reported with use of bundle approaches. For example, Eggimann et al. measured the long-term impact of a multimodal strategy to decrease CLA-BSI
in the ICUs of the University of Geneva Hospitals (172). The incidence of CLA-BSI has remained low since the implementation of a bundle approach, decreasing by 79% over the study period and by 75% after 6 years; however, probably best known is the recent work of Pronovost et al. (173,174). This collaborative cohort study, predominantly among ICUs of Michigan hospitals, implemented an evidenced-based intervention to reduce CLA-BSIs (173). The median rate of CLA-BSI per 1,000 catheter-days decreased from 2.7 infections at baseline to 0 at 3 months after implementation (p < .002), and the mean rate per 1,000 catheter-days decreased from 7.7 at baseline to 1.4 at 16 to 18 months of follow-up (p



in the ICUs of the University of Geneva Hospitals (172). The incidence of CLA-BSI has remained low since the implementation of a bundle approach, decreasing by 79% over the study period and by 75% after 6 years; however, probably best known is the recent work of Pronovost et al. (173,174). This collaborative cohort study, predominantly among ICUs of Michigan hospitals, implemented an evidenced-based intervention to reduce CLA-BSIs (173). The median rate of CLA-BSI per 1,000 catheter-days decreased from 2.7 infections at baseline to 0 at 3 months after implementation (p < .002), and the mean rate per 1,000 catheter-days decreased from 7.7 at baseline to 1.4 at 16 to 18 months of follow-up (p
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree