The Immune System
William E. Paul
The immune system is a remarkable defense mechanism. It makes rapid, specific, and protective responses against the myriad potentially pathogenic microorganisms that inhabit the world in which we live. The tragic examples of acquired immunodeficiency syndrome (AIDS) and the inherited severe combined immunodeficiencies graphically illustrate the consequences of a nonfunctional adaptive immune system. Patients with AIDS and children with severe combined immunodeficiency often fall victim to infections that are of little or no consequence to those with normally functioning immune systems. The immune system also has a role in the rejection of tumors and, when dysregulated, may give rise to a series of autoimmune diseases, including insulin-dependent diabetes mellitus, multiple sclerosis, rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease, among others.
Fundamental Immunology has as its goal the authoritative presentation of the basic elements of the immune system, of the means through which the mechanisms of immunity act in a wide range of clinical conditions, including recovery from infectious diseases, rejection of tumors, transplantation of tissue and organs, autoimmunity and other immunopathologic conditions, and allergy, and how the mechanisms of immunity can be marshaled by vaccination to provide protection against microbial pathogens.
The purpose of the opening chapter is to provide readers with a general introduction to our current understanding of the immune system. It should be of particular importance for those with a limited background in immunology, providing them with the preparation needed for subsequent chapters of the book. Rather than providing extensive references in this chapter, each of the subject headings will indicate the chapters that deal in detail with the topic under discussion. Those chapters will not only provide an extended treatment of the topic but will also furnish the reader with a comprehensive reference list that can be found in the online version of Fundamental Immunology.
KEY CHARACTERISTICS OF THE IMMUNE SYSTEM
Innate Immunity (Chapters 15, 17, 19, and 20)
Powerful nonspecific defenses prevent or limit infections by most potentially pathogenic microorganisms. The epithelium provides both a physical barrier to the entry of microbes and produces a variety of antimicrobial factors. Agents that penetrate the epithelium are met with macrophages and related cells possessing “microbial sensors” that recognize key molecules characteristic of many microbial agents. These “pattern recognition receptors” include several families of molecules, of which the most intensively studied are the toll-like receptors (TLRs) and the nucleotide oligomerization domain-like receptors. Each TLR recognizes a distinct substance (or set of substances) associated with microbial agents; for example, TLR4 recognizes lipopolysaccharides, TLR3, doublestranded ribonucleic acid, and TLR9, unmethylated CpG-containing DNA. Because the recognized substances are generally indispensable to the infectious agent, microbial sensors provide a highly efficient means to recognize potential pathogens.
The interaction of a TLR with its ligand induces a series of intracellular signaling events, of which activation of the NF-κB system is particularly important. Macrophage activation with enhancement of the cell’s phagocytic activity and the induction of antimicrobial systems aid in the destruction of the pathogen. The induction of an inflammatory response as a result of the activation of the innate immune system recruits other cell types, including neutrophils, to the site. The innate system can provide an effective means to control or eliminate pathogens. Indeed, life forms other than vertebrates rely on the innate immune system to allow them to deal with microbial infection.
In vertebrates, the innate immune system also acts to recruit antigen-specific immune responses, not only by attracting cells of the immune system to the site of infection, but also through the uptake of antigen by dendritic cells (DCs) and its transport by these cells to lymphoid tissues where primary immune responses are initiated. Activated DCs express cell surface costimulatory molecules and produce cytokines that can regulate the quality of the immune response so that it is most appropriate to combating the particular infectious agent, be it a virus, bacterium, or parasite.
Primary Responses (Chapters 10 and 14)
Primary immune responses are initiated when a foreign antigenic substance interacts with antigen-specific lymphocytes under appropriate circumstances. The response generally consists of the production of antibody molecules specific for the antigenic determinants of the immunogen and of the expansion and differentiation of antigen-specific helper and effector T-lymphocytes. The latter include cytokine-producing cells and killer T cells, capable of lysing infected cells. Generally, the combination of the innate immune response and the primary adaptive response are sufficient to eradicate or to control the microbe. Indeed, the most effective function of the immune system is to mount a response that eliminates the infectious agent from the body, so-called sterilizing immunity.
Secondary Responses and Immunologic Memory (Chapters 10, 14, 29, and 31)
As a consequence of initial encounter with antigen, the immunized individual develops a state of immunologic memory. If the same (or a closely related) microorganism is encountered again, a secondary response is made. This generally consists of an antibody response that is more rapid, greater in magnitude, and composed of antibodies that bind to the antigen with greater affinity and are more effective in clearing the microbe from the body. A more rapid and more effective T-cell response also ensues. Thus, an initial infection with a microorganism often initiates a state of immunity in which the individual is protected against a second infection. In the majority of situations, protection is provided by high-affinity antibody molecules that rapidly clear the re-introduced microbe. This is the basis of most licensed vaccines; the great power of vaccines is illustrated by the elimination of smallpox from the world and by the complete control of polio in the western hemisphere.
The Immune Response is Highly Specific and the Antigenic Universe is Vast
The immune response is highly specific. Primary immunization with a given microorganism evokes antibodies and T cells that are specific for the antigenic determinants found on that microorganism but that generally fail to recognize (or recognize only poorly) antigenic determinants expressed by unrelated microbes. Indeed, the range of antigenic specificities that can be discriminated by the immune system is enormous.
The Immune System is Tolerant of Self-Antigens (Chapters 32 and 33)
One of the most important features of the immune system is its ability to discriminate between antigenic determinants expressed on foreign substances, such as pathogenic microbes, and potential antigenic determinants expressed by the tissues of the host. The failure of the system to make full-blown immune responses to self-antigens is referred to as immunologic tolerance. Tolerance is a complex process that actually involves several distinct processes. One element, perhaps the most important, is an active process involving the elimination or inactivation of cells that can recognize self-antigens. In addition, there are mechanisms through which cells that encounter antigens (such as selfantigens) in the absence of cues from the innate immune system may fail to make a response, may make a minimal response, or may be inactivated through a process referred to as anergy. Finally, a specialized set of T cells exist designated regulatory cells that actively suppress responses against self-antigens. Indeed, individuals who have mutations in the key transcription factor Foxp3 expressed by the regulatory cells develop severe multiorgan autoimmunity (Immunodysregulation polyendocrinopathy, enteropathy X-linked syndrome). The critical necessity to control selfreactivity is clearly shown by this multilayered system that involves elimination, inactivation, and suppression.
Immune Responses Against Self-Antigens can Result in Autoimmune Diseases (Chapter 44)
Failure in establishing immunologic tolerance or unusual presentations of self-antigens can give rise to tissuedamaging immune responses directed against antigenic determinants on host molecules. These can result in autoimmune diseases. As has already been mentioned, a group of extremely important diseases are caused by autoimmune responses or have major autoimmune components, including systemic lupus erythematosus, rheumatoid arthritis, insulin-dependent diabetes mellitus, multiple sclerosis, myasthenia gravis, and inflammatory bowel disease. Efforts to treat these diseases by modulating the autoimmune response are a major theme of contemporary medicine.
Acquired Immunodeficiency Syndrome is an Example of a Disease Caused by a Virus That the Immune System Generally Fails to Eliminate (Chapter 42)
Immune responses against infectious agents do not always lead to elimination of the pathogen. In some instances, a chronic infection ensues in which the immune system adopts a variety of strategies to limit damage caused by the organism or by the immune response. Indeed, herpes viruses, such as human cytomegalovirus, frequently are not eliminated by immune responses and establish a chronic infection in which the virus is controlled by immune responses. One of the most notable infectious diseases in which the immune response generally fails to eliminate the organism is AIDS, caused by the human immunodeficiency virus (HIV). In this instance, the principal infected cells are those of the immune system
itself, leading to an eventual state in which the individual can no longer mount protective immune responses against other microbial pathogens. Indeed, under the assault of HIV, control of viruses such as cytomegalovirus is lost and they may cause major tissue damage.
itself, leading to an eventual state in which the individual can no longer mount protective immune responses against other microbial pathogens. Indeed, under the assault of HIV, control of viruses such as cytomegalovirus is lost and they may cause major tissue damage.
Major Principles of Immunity
The major principles of the immune response are:
Elimination of many microbial agents through the nonspecific protective mechanisms of the innate immune system
Cues from the innate immune system inform the cells of the adaptive immune system as to whether it is appropriate to make a response and what type of response to make
Cells of the adaptive immune system display exquisitely specific recognition of foreign antigens and mobilize potent mechanisms for elimination of microbes bearing such antigens
The immune system displays memory of its previous responses
Tolerance of self-antigens.
The remainder of this introductory chapter will describe briefly the molecular and cellular basis of the system and how these central characteristics of the immune response may be explained.
CELLS OF THE IMMUNE SYSTEM AND THEIR SPECIFIC RECEPTORS AND PRODUCTS
The immune system consists of several distinct cell types, each with important roles. The lymphocytes occupy central stage because they are the cells that determine the specificity of immunity. It is their response that orchestrates the effector limbs of the immune system. Cells that interact with lymphocytes play critical parts both in the presentation of antigen and in the mediation of immunologic functions. These cells include DCs and the closely related Langerhans cells, monocyte/macrophages, natural killer (NK) cells, neutrophils, mast cells, basophils, and eosinophils. In addition, a series of specialized epithelial and stromal cells provide the anatomic environment in which immunity occurs, often by secreting critical factors that regulate migration, growth and homeostasis, and gene activation in cells of the immune system. Such cells also play direct roles in the induction and effector phases of the response.
The cells of the immune system are found in peripheral organized tissues, such as the spleen, lymph nodes, Peyer’s patches of the intestine, and tonsils, where primary immune responses generally occur (see Chapter 3). Many of the lymphocytes comprise a recirculating pool of cells found in the blood and lymph, as well as in the lymph nodes and spleen, providing the means to deliver immunocompetent cells to sites where they are needed and to allow immunity that is initiated locally to become generalized. Activated lymphocytes acquire the capacity to enter nonlymphoid tissues where they can express effector functions and eradicate local infections. Some memory lymphocytes are “on patrol” in the tissues, scanning for reintroduction of their specific antigens. Lymphocytes are also found in the central lymphoid organs, the thymus, and bone marrow, where they undergo the developmental steps that equip them to mediate the responses of the mature immune system.
Individual lymphocytes are specialized in that they are committed to respond to a limited set of structurally related antigens. This commitment exists before the first contact of the immune system with a given antigen. It is expressed by the presence on the lymphocyte’s surface of receptors specific for determinants (epitopes) of the antigen. Each lymphocyte possesses a population of receptors, all of which have identical combining sites (this is a slight oversimplification as occasionally T cells and less frequently B cells may express two populations of receptors). One set, or clone, of lymphocytes differs from another clone in the structure of the combining region of its receptors and thus in the epitopes that it can recognize. The ability of an organism to respond to virtually any non-self-antigen is achieved by the existence of a very large number of different lymphocytes, each bearing receptors specific for a distinct epitope. As a consequence, lymphocytes are an enormously heterogeneous group of cells. Based on reasonable assumptions as to the range of diversity that can be created in the genes encoding antigen-specific receptors, it is virtually certain that the number of distinct combining sites on lymphocyte receptors of an adult human can be measured in the millions.
Lymphocytes differ from each other not only in the specificity of their receptors but also in their functions. There are two broad classes of lymphocytes: the B-lymphocytes, which are precursors of antibody-secreting cells, and the T (thymus-derived)- lymphocytes. T-lymphocytes express important helper functions, such as the ability to aid in the development of specific types of immune responses, including the production of antibody by B cells, the increase in the microbicidal activity of macrophages, and the recruitment of granulocytes to sites of infection. Other T-lymphocytes are involved in direct effector functions, such as the lysis of virus-infected cells or certain neoplastic cells. Regulatory T-lymphocytes have the capacity to suppress immune responses.
B-LYMPHOCYTES AND ANTIBODY
B-Lymphocyte Development (Chapter 8)
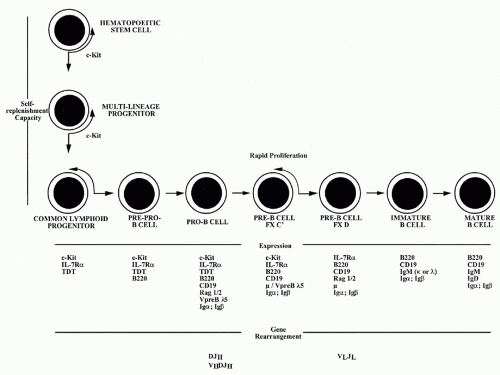
B-lymphocytes derive from lymphoid progenitor cells, which in turn are derived from hematopoietic stem cells (Fig. 1.1). A detailed picture has been obtained of the molecular mechanisms through which committed early members of the B lineage develop into mature B-lymphocytes. These events occur in the fetal liver and, in adult life, in the bone marrow. Interaction with specialized stromal cells and their products, including cytokines such as interleukin (IL)-7 and BAFF, are critical to the normal regulation of this process.
The key events in B-cell development involve commitment to the B lineage and repression of the capacity to differentiate to cells of other lineages. In pro-B cells and pre-B cells, the genetic elements that encode the antigen-specific receptors are assembled. These receptors are im
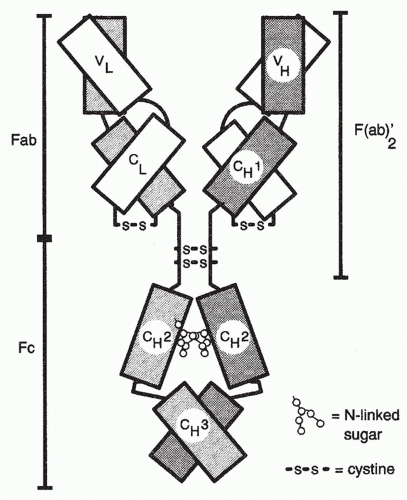
munoglobulin (Ig) molecules specialized for expression on the cell surface. Igs are heterodimeric molecules consisting of heavy (H) and light (L) chains, both of which have variable (V) regions, which are responsible for the binding of antigen and that differ in sequence from one Ig molecule to another (see Chapters 5, 6, and 7) (Fig. 1.2) and constant (C) regions.
munoglobulin (Ig) molecules specialized for expression on the cell surface. Igs are heterodimeric molecules consisting of heavy (H) and light (L) chains, both of which have variable (V) regions, which are responsible for the binding of antigen and that differ in sequence from one Ig molecule to another (see Chapters 5, 6, and 7) (Fig. 1.2) and constant (C) regions.
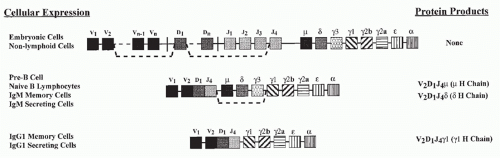
The genetic elements encoding the variable portions of Ig H and L chains are not contiguous in germline DNA or in the DNA of nonlymphoid cells (see Chapter 6) (Fig. 1.3). In pro- and pre-B cells, these genetic elements are translocated to construct an expressible V-region gene. This process involves a choice among a large set of potentially usable V, diversity (D), and joining (J) elements in a combinatorial manner and depends upon the recombinating activating gene (RAG) proteins, RAG1 and RAG2. Such combinatorial translocation, together with the addition of diversity in the course of the joining process, results in the generation of a very large number of distinct H and L chains. The pairing of H and L chains in a quasirandom manner further expands the number of distinct Ig molecules that can be formed.
The H-chain V region is initially expressed in association with the product of the µC-region gene. Together, these elements encode the µ IgH chain, which is used in Igs of the IgM class.
The successful completion of the process of Ig gene rearrangement and the expression of the resultant IgM on the cell surface marks the transition between the pre-B- and B-cell states (see Fig. 1.1). The newly differentiated B cell initially expresses surface Ig solely of the IgM class. The cell completes its maturation process by expressing on its surface a second class of Ig composed of the same L chain and the same H chain V (VDJ) region but of a different H chain C region; this second Ig H chain is designated δ, and the Ig to which it contributes is designated IgD, so that the mature naïve B cells express both IgM and IgD surface molecules that share the same V region.
The differentiation process is controlled at several steps by a system of checks that determines whether prior steps have been successfully completed. These checks depend on the expression on the surface of the cell of appropriately constructed Ig or Ig-like molecules. For, example,
in the period after a µ chain has been successfully assembled but before an L chain has been assembled, the µ chain is expressed on the cell surface in association with a surrogate L chain, consisting of VpreB and λ5. Pre-B cells that fail to express this µ/VpreB λ5 complex do not move forward to future differentiation states or do so very inefficiently.
in the period after a µ chain has been successfully assembled but before an L chain has been assembled, the µ chain is expressed on the cell surface in association with a surrogate L chain, consisting of VpreB and λ5. Pre-B cells that fail to express this µ/VpreB λ5 complex do not move forward to future differentiation states or do so very inefficiently.
B-Lymphocyte Activation (Chapter 9)
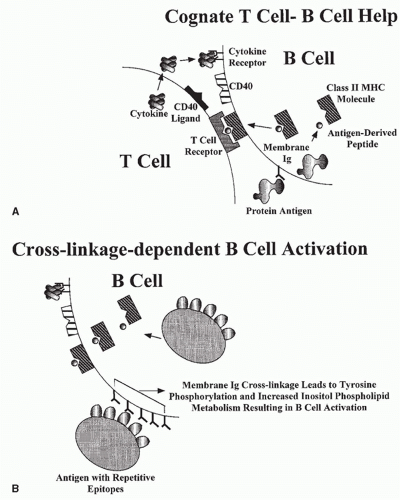
A mature B cell can be activated by an encounter with antigen-expressing epitopes that are recognized by its cell surface Ig (Fig. 1.4). The activation process may be a direct one, dependent on cross-linkage of membrane Ig molecules by the antigen (cross-linkage-dependent B-cell activation), or an indirect one, occurring most efficiently in the context of an intimate interaction with a helper T cell, in a process often referred to as cognate help.
Because each B cell bears membrane Ig molecules with identical variable regions, cross-linkage of the cell surface receptors requires that the antigen express more than one copy of an epitope complementary to the binding site of the receptor. This requirement is fulfilled by antigens with repetitive epitopes. Among these antigens are the capsular polysaccharides of many medically important microorganisms such as pneumococci, streptococci, and meningococci. Similar expression of multiple identical epitopes on a single immunogenic particle is a property of many viruses because they express multiple copies of envelope proteins on their surface. Cross-linkage-dependent B-cell activation is a major protective immune response mounted against these microbes. The binding of complement components (see Chapter 36) to antigen or antigen/antibody complexes can increase the magnitude of the cross-linkage-dependent B-cell activation due to the action of a receptor for complement, which, together with other molecules, increases the magnitude of a B-cell response to limiting amounts of antigen.
Cognate help allows B cells to mount responses against antigens that cannot cross-link receptors and, at the same time, provides costimulatory signals that rescue B cells from inactivation when they are stimulated by weak cross-linkage events. Cognate help is dependent on the binding of antigen by the B cell’s membrane Ig, the endocytosis of the antigen, and its fragmentation into peptides within the endosomal/lysosomal compartment of the cell. Some of the resultant peptides are loaded into a groove in a specialized set of cell surface proteins, the class II major histocompatibility complex (MHC) molecules (Fig. 1.5). The resultant class II/
peptide complexes are expressed on the cell surface. As will be discussed subsequently, these complexes are the ligands for the antigen-specific receptors of a set of T cells designated CD4 T cells. CD4 T cells that have receptors specific for the class II/peptide complex expressed on the B-cell surface recognize and interact with that B cell. That interaction results in the activation of the B cell through the agency of cell surface molecules expressed by the T cells (e.g., the CD40 ligand [CD154]) and cytokines produced by the T cell (see Fig. 1.4). The role of the B-cell receptor for antigen is to create the T-cell ligand on the surface of antigen-specific B cells; activation of the B cell derives largely from the action of the T cell. However, in many physiologic situations, receptor cross-linkage stimuli and cognate help synergize to yield more vigorous B-cell responses. Recently, it has been shown that the association of ligands for TLRs with antigen will strikingly enhance B cell responses.
peptide complexes are expressed on the cell surface. As will be discussed subsequently, these complexes are the ligands for the antigen-specific receptors of a set of T cells designated CD4 T cells. CD4 T cells that have receptors specific for the class II/peptide complex expressed on the B-cell surface recognize and interact with that B cell. That interaction results in the activation of the B cell through the agency of cell surface molecules expressed by the T cells (e.g., the CD40 ligand [CD154]) and cytokines produced by the T cell (see Fig. 1.4). The role of the B-cell receptor for antigen is to create the T-cell ligand on the surface of antigen-specific B cells; activation of the B cell derives largely from the action of the T cell. However, in many physiologic situations, receptor cross-linkage stimuli and cognate help synergize to yield more vigorous B-cell responses. Recently, it has been shown that the association of ligands for TLRs with antigen will strikingly enhance B cell responses.
B-Lymphocyte Differentiation (Chapters 9 and 10)
Activation of B cells prepares them to divide and to differentiate either into antibody-secreting cells or into memory cells, so that there are more cells specific for the antigen used for immunization. Those cells that differentiate into antibody-secreting cells account for primary antibody responses. Some of these antibody-secreting cells migrate to the bone marrow where they may continue to produce antibody for an extended period of time and may have lifetimes very long.
Memory B cells give rise to antibody-secreting cells upon rechallenge of the individual. The hallmark of the antibody response to rechallenge (a secondary response) is that it is of greater magnitude, occurs more promptly, is composed of antibodies with higher affinity for the antigen, and is dominated by Igs expressing γ, α, or ε C regions (IgG, IgA, or IgE) rather than by IgM, which is the dominant Ig of the primary response.
Division and differentiation of cells into antibody-secreting cells is largely controlled by the interaction of the activated B cells with T cells expressing CD154 and by their stimulation by T-cell-derived cytokines.
The differentiation of activated B cells into memory cells occurs in a specialized microenvironmental structure in the spleen and lymph nodes: the germinal center. The increase in antibody affinity also takes place within the germinal center. This process, designated affinity maturation, is dependent on somatic hypermutation. The survival of B cells within the germinal center depends on their capacity to bind antigen so that as the amount of antigen diminishes, B cells that have higher affinity receptors, either naturally or as a result of the hypermutation process, have a selective survival and growth advantage. Thus, such cells come to dominate the population.
The process through which a single H-chain V region can become expressed with genes encoding C regions other than µ or δ is referred to as Ig class switching. It is dependent on a gene translocation event through which the C-region genes between the genetic elements encoding the V region and the newly expressed C gene are excised, resulting in the switched C gene being located in the position that the Cµ gene formerly occupied (see Fig. 1.3). This process also occurs mainly in germinal centers. Both somatic hypermutation and immunoglobulin class switching depend upon the action of activation-induced cytidine deaminase (AID) that plays an important role in the breakage and repair of DNA, which is essential for recombination events.
B1 and Marginal Zone B-Lymphocytes (Chapters 8 and 10)
B lymphocytes consist of at least three distinct populations: conventional B cells, B1 B cells, and marginal zone B cells. B1 B cells were initially recognized because some express a cell-surface protein, CD5, not generally found on other B cells. In the adult mouse, B1 B cells are found in relatively high frequency in the peritoneal cavity but are present at low frequency in the spleen and lymph nodes. B1 B cells are quite numerous in fetal and perinatal life and appear to be self-renewing, in contrast to conventional B cells, in which division and memory are antigen driven.
Marginal zone B cells are localized in a distinct anatomical region of the spleen (the marginal zone) that represents the major antigen filtering and scavenging area. Like B1 B cells, marginal zone B cells express a repertoire biased toward bacterial cell wall constituents and senescent self-components. Marginal zone and B1 B cells respond very rapidly to antigenic challenge, likely independently of T cells. Uniquely, among all populations of B cells, marginal zone B cells are dependent on Notch-2 signaling for their development.
B1 B cells and marginal zone B cells are responsible for the secretion of the serum IgM that exists in nonimmunized mice, often referred to as natural IgM. Among the antibodies found in such natural IgM are molecules that can combine with phosphatidylcholine (a component of pneumococcal cell walls) and with lipopolysaccharide and influenza virus. B1 B cells also produce autoantibodies, although they are generally of low affinity and in most cases not pathogenic. There is evidence that B1 B cells are important in resistance to several pathogens and may have a significant role in mucosal immunity.
B-Lymphocyte Tolerance (Chapter 32)
One of the central problems facing the immune system is that of being able to mount highly effective immune responses to the antigens of foreign, potentially pathogenic agents while ignoring antigens associated with the host’s own tissues. The mechanisms ensuring this failure to respond to self-antigens are complex and involve a series of strategies. Chief among them is elimination of cells capable of self-reactivity or the inactivation of such cells. The encounter of immature, naïve B cells with antigens with repetitive epitopes capable of cross-linking membrane Ig can lead to elimination of the B cells, particularly if no T-cell help is provided at the time of the encounter. This elimination of potentially self-reactive cells is often referred to as clonal elimination. Many selfreactive cells, rather than dying upon encounter with selfantigens, undergo a further round of Ig gene rearrangement. This receptor editing process allows a self-reactive cell to substitute a new receptor and therefore to avoid elimination.
There are many self-antigens that are not encountered by the developing B-cell population or that do not have the capacity to cross-link B-cell receptors to a sufficient degree to elicit the receptor editing /clonal elimination process. Such cells, even when mature, may nonetheless be inactivated through a process that involves cross-linkage of receptors without the receipt of critical costimulatory signals. These inactivated cells may be retained in the body but are unresponsive to antigen and are referred to as anergic. When removed from the presence of the anergy-inducing stimulus, anergic cells may regain responsiveness.
Immunoglobulins
Structure (Chapter 5)
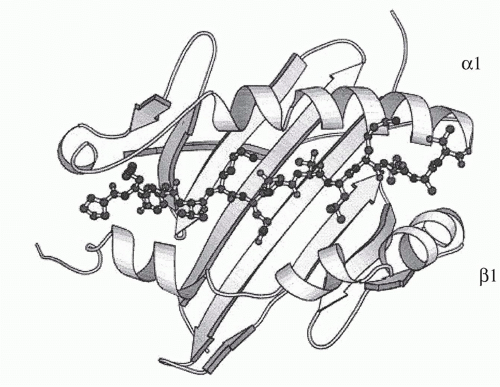
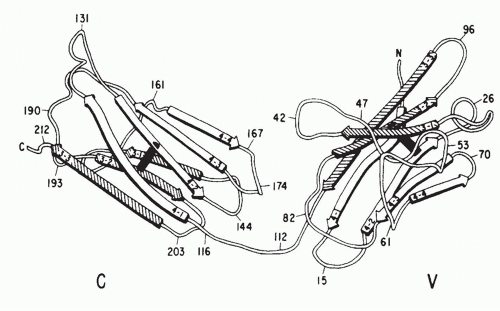
Igs are the antigen-specific membrane receptors and secreted products of B cells. They are members of a large family of proteins designated the Ig supergene family. Members of the Ig supergene family have sequence homology, a common gene organization, and similarities in three-dimensional structure. The latter is characterized by a structural element referred to as the Ig fold, generally consisting of a set of seven β-pleated sheets organized into two opposing layers (Fig. 1.6). Many of the cell surface proteins that participate in immunologic recognition processes, including the T-cell receptor (TCR), the CD3 complex, and signaling molecules associated with the B-cell receptor (Igα and Igβ), are members of the Ig supergene family.
The Igs themselves are constructed of a unit that consists of two H chains and two L chains (see Fig. 1.2). The H and L chains are composed of a series of domains, each consisting of approximately 110 amino acids.
The L chains, of which there are two types (κ and λ), consist of two domains. The carboxy-terminal domain is essentially identical among L chains of a given type and is referred to as the C region. As already discussed, the amino terminal domain varies from L chain to L chain and contributes to the binding site of antibody. Because of its variability, it is referred to as the V region. The variability of this region is largely concentrated in three segments, designated the hypervariable or complementarity-determining regions (CDRs). The CDRs contain the amino acids that are the L chain’s contribution to the lining of the antibody’s combining site. The three CDRs are interspersed among four regions of much lower degree of variability, designated framework regions.
The H chains of Ig molecules are of several classes determined by their constant regions (µ, δ, γ [of which there are several subclasses], α and ε). An assembled Ig molecule, consisting of one or more units of two identical H and L chains, derives its name from the constant region of the H chain that it possesses. Thus, there are IgM, IgD, IgG, IgA, and IgE antibodies. The H chains each consist of a single amino terminal V region and three or four C regions. In many H chains, a hinge region separates the first and second C regions and conveys flexibility to the molecule, allowing the two combining sites of a single unit to move in relation to one another so as to promote the binding of a single antibody molecule to an antigen that has more than one copy of the same epitope. Such divalent binding to a single antigenic structure results in a great gain in energy of interaction (see Chapter 7). The H-chain V region, like that of the L chain, contains three CDRs lining the combining site of the antibody and four framework regions.
The C region of each H-chain class conveys unique functional attributes to the antibodies that possess it. Among the distinct biologic functions of each class of antibody are the following:
IgM antibodies are potent activators of the complement system (see Chapter 36).
IgA antibodies are secreted into a variety of bodily fluids and are principally responsible for immunity at mucosal surfaces (see Chapter 34).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree