A—No motor or sensory function below the level of the lesion
B—No motor function below the level of the lesion but sensory function that continues into the sacral segments
C—Most motor function below the level of the lesion preserved, and more than half of key muscles have a motor grade of 3 or less
D—Most motor function below the level of the lesion preserved, and more than half of key muscles have a motor grade of greater than 3
E—Normal motor and sensory function
The neurological recovery below the level of SCI is possible, but patients in Asia A generally show no significant recovery (5% or less) in this area a year after SCI. Individuals who are classified ASIA B have approximately a 35% chance of improving to grade C or D and almost none (statistically significant) to grade E 1 year after the SCI. Instead, those classified initially with grades C can improve to grade D with a likelihood of 60–70% [7].
In addition to physical examination, MRI examination of the spinal cord can provide information regarding future recovery. Hemorrhage within the cord is suggestive of an unfavorable prognosis, with a better prognosis for improvement suggested by (in order) contusion, edema, and a normal appearance of the cord.
39.3 An Onset View of the SCI in the Elderly and Its Medical Complications Resulting Therefrom
Early onset SCI differs from late onset SCI in elderly patients can occur in (A) those injured when young but with a long history of SCI who then have reached old age, known as early onset, and (B) those whose SCI onset have arrived when senior, known as late onset (see Table 39.2). While both types can be the result of TSCI or NTSCI causes, TSCI happens less frequently in older patients in whom SCI is a consequence of falls or motor vehicle accident (MVA).
Table 39.2
Elderly with spinal cord injury and clinical onset
Early onset | Late onset | |
|---|---|---|
1. SCI? | Injured young, long history of SCI, have reached old age | Those whose SCI onset have arrived when senior |
2. Physical and physiologic declines | SCI onset ≤15 years old, the maintenance phase last ≥20 years SCI onset ≥20 years old, the functional decline phase is “quick” and aging speed at a much younger age | The acute phase can last longer, the maintenance phase is short, and the functional decline phase is “quick” |
3. SCI perpetuating degenerative effects | Present | Present |
4. Physical capacity reduction | Reduced | Incredibly reduced |
5. Pneumonia, kidney stone, decubitus ulcer | Often in tetraplegia | Often in paraplegia/tetraplegia |
6. Premorbid medical conditions | Is rare, minor | Frequent and complicated |
Early Onset
SCI elderly by early onset differ from SCI seniors from late onset. In this sense, SCI can exacerbate the physical and physiologic declines—including in the musculoskeletal system, cardiovascular, gastrointestinal (GI), pulmonary, and integumentary accompanied by the aging process. A number of long-term follow-up studies and many authors have documented the tendency for individuals with SCI to age more rapidly than the population of able-bodied. That means, individuals with SCI develop characteristics and medical problems commonly associated with the aging process at a much younger age. Kempt talking about Long-Term Outcomes with Disability, during the Rancho Los Amigos Seminar in 1998, said that younger individuals whose SCI onset was during or prior to adolescence may enjoy a maintenance phase of 20 years prior to experiencing functional decline (see Fig. 39.1).
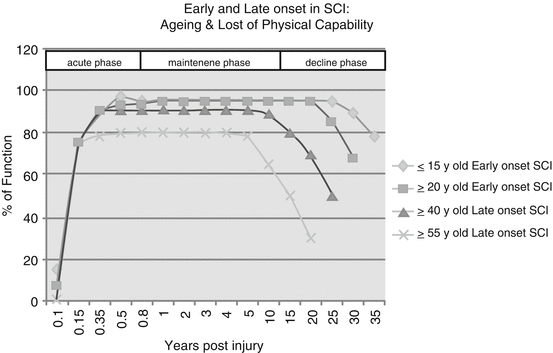

Fig. 39.1
Aging and loss of physical capability (capacity) in SCI individuals
Cushman and Hassett found that, in people with SCI of 15 years or more, 93% of them had experienced a decline in functional status [8]. A study by Smith et al. found that in adults with long-term disability—specifically, SCI, multiple sclerosis, muscular dystrophy, or post-polio syndrome—the development of chronic comorbid medical conditions is associated with factors such as body mass index, waist circumference, and the existence of another chronic comorbidity [9].
Late Onset
According to Kempt (Rancho Los Amigos Seminar 1998), individuals who are aged 55 years at the onset of their SCI may only have 5–7 years of relatively stable functioning status prior to experiencing a decline. In fact older people may have premorbid medical conditions associated that can determine poorer end results of the rehabilitation process. In one US study conducted during the 1970s and 1980s, 24.3% of persons with SCI who were above 60 years old at injury had arthritis, 8.6% had significant heart disease, 4.3% had diabetes, and 4.3% were obese [10, 11]. Above age 61, there is a greater risk of diabetes, heart disease, obesity and arthritis, poorer outcomes with respect to walking, and more difficulties with bladder and bowel independence. Long-term medical complication rates increase with both older age and greater injury severity [12–16]. During the 5th post-injury year, among persons who are >60 years of age, 7.1% develop pneumonia, and 29.5% have abnormal renal function. The corresponding figures for persons >40 years of age are 2.2% for pneumonia and 10.2% for abnormal renal function. The long-term odds of developing a kidney stone are 50% higher for persons who are at least 55 years of age compared with those who are aged 25–34 and 90% higher for persons with complete tetraplegia compared with persons with AIS D injuries [13]. There is also an increased risk of pressure ulcers among the elderly, and they play an important role in rehabilitation outcomes [17–19]. The odds of developing a pressure ulcer are 30% higher among persons who are at least 50 years of age compared with those who are aged 15–29 [20]. However, advanced age appears to be related not only to the frequency of pressure ulcers but also to their severity. In fact an aging-related decrease in muscle mass and vascularity may reduce the tolerance of aged skin to pressure and shearing forces, consequently leading to the development of pressure ulcers [21]. In any case, the vast majority of young and/or seniors with SCI after discharge from an intensive rehabilitation center can become sedentary on a wheelchair, not participating much in physical activities [22, 23], exposed to severe degenerative effects that perpetuate (see Table 39.3).
Despite the age of SCI onset, a consequent physical capacity (capability) reduction, like in the sedentary lifestyle from SCI, is a condition that definitively can offer health deterioration, increasing the risk of medical complications secondary to a chronic disability status [34]. It’s known that the physical capability in individuals with spinal cord injury is directly related to independence in ADLs (activities of daily living), and it’s extremely necessary to keep the physical capacity of these individuals after discharge. In contrary, self-independence in a wheelchair is not enough to keep the patient healthy. Studies on wheelchair users with SCI indicated that those who maintain a more active lifestyle by regularly participating in exercise and sports programs can increase their muscle strength, aerobic fitness, and physical performance to levels well above those of their sedentary cohorts [45–48]. As in the able-bodied population, physical capability for SCI appears to decline with age [10]. Among SCI in general and in particular in persons >60 years of age at injury, 47% had at least one pressure sore during initial hospitalization, 30% developed pneumonia, 11.4% had deep vein thrombosis, 10% had a gastrointestinal hemorrhage, and 5.7% had a renal stone [10, 11] (see Table 39.4).
Table 39.4
Secondary medical complications in SCI
Thromboembolic disease: DVT Decubitus ulcer |
Autonomic dysfunction: • Orthostatic hypotension • Bradycardia • Autonomic dysreflexia |
Neurogenic bladder and sex dysfunction |
Neurogenic bowel dysfunction |
Heterotopic ossification Spasticity Depression |
39.4 Effects of Aging in Spinal Cord Injury
The aging process for some groups of people with disabilities begins earlier than usual. Some people with developmental disabilities show signs of premature aging in their 40s and 50s [21], and they may experience age-related health conditions more frequently. The aging process and associated changes (presbycusis, deconditioning, loss of strength and balance, osteoporosis) may have a greater impact on people with disabilities. Those with existing mobility impairments (like in SCI individuals) may increasingly experience functional loss as they age [49] (see Fig. 39.1). Generally speaking, normal aging is associated with a reduction in functional reserve capacity in tissues and organs. The following are some of the physiological changes that may be expected with aging in the SCI population:
Cardiovascular Changes
The incidence of cardiovascular disease among individuals with SCI is over 200% higher than the expected incidence in an age- and gender-matched control population (Kocina et al.) [50]. In individuals surviving 30 years or longer following SCI, nearly 50% of all deaths occur due to premature cardiovascular disease (CVD) [51].
Besides the autonomic dysfunction in SCI, hypertension, HDL decrease, reduced exercise tolerance, platelet aggregation, obesity, and reduced venous return (due to decrease in sympathetic tone and a reduction in the muscular pumping action of the lower extremities) are some of the causes that lead to myocardial infarcts and diabetes in elderly SCI individuals. Up to 75% of the SCI population are overweight or obese [52] and have adipose tissue deposited particularly around the abdomen, increasing the risk for developing CVD. Disruption of the autonomic nervous system and from the normal cardiovascular control mechanisms also contributes to heightened cardiovascular risk, with changes to peripheral vasculature, blood pressure abnormalities, heart rate variability, occurrence of cardiac arrhythmias, and a blunted cardiovascular response to exercise, limiting the capacity to perform physical activity [53].
Pulmonary Changes
Following SCI, respiratory complications are the most common cause of death [54–57]. The spectrum of pulmonary complications following SCI includes respiratory failure, pneumonia, atelectasis, pulmonary thromboembolism, sleep apnea disorders, dyspnea, and dysphonia. But, what are the pathophysiological issues that are at the basis of respiratory problems in spinal cord injury? In these patients, the reduction in lung compliance was attributed to reduced lung volume and changes in the mechanical properties of the lung due to alterations in the surfactant, which can occur quickly with low lung volume ventilation [56]. The inefficiency of the respiratory system contributes to the risk of respiratory muscle fatigue, particularly during pneumonia and/or airway obstruction. During inspiratory resistive-loaded inhalation in tetraplegia, the causes of inefficiency during the inspiratory acts are due to the decrease of the lower transverse dimension of the rib cage; the diaphragm does not work close to its optimal length for the tension production. In complete C2 SCI and below, there is a reduction of predicted vital capacity (VC) of 20–50%, inefficiency in ventilation, and markedly impaired cough, due to changes in lung compliance, chest-wall distortion, and impairment in both muscles of inhalation and exhalation.
Diminished pulmonary function can result from restrictive disease, obstructive disease, or a combination of these. In spinal cord injury (SCI), restrictive lung disease occurs as a result of respiratory muscle paralysis. The higher the level of SCI, the greater the restrictive impairment. Weakened muscles of inhalation prevent deep breaths, and some patients with quadriplegia may not sigh, leading to atelectasis and related gas-exchange and lung-compliance abnormalities. Dysfunctional muscles of exhalation cause impaired cough and secretion clearance (with associated atelectasis), increase in airways resistance, and persistence of infection when it occurs [56] (see Fig. 39.2).
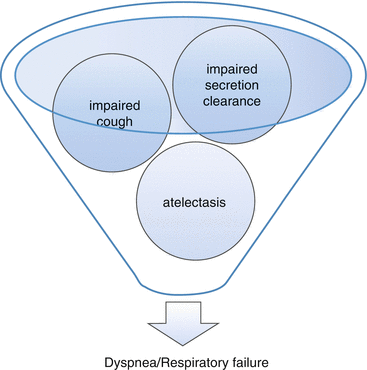

Fig. 39.2
Dysfunctional muscles of exhalation in SCI can cause respiratory failure
The development of kyphosis, scoliosis, or increasing spasticity can cause further restrictive disease as the individual with SCI ages.
Sleep apnea reportedly occurs in 40% of individuals with SCI. The frequency of obstructive sleep apnea increases with age. Of those individuals with SCI who experience sleep apnea, only 25% have been found to be obese. The long-term use of baclofen may be associated with the development of obstructive sleep apnea.
Neurological Changes
The peripheral nervous system (PNS) acts as the trait d’union for communication between the central nervous system (CNS)/brain and the limbs, organs, and tissues of the rest of the body, transmitting signals to coordinate actions. The reaction time which these signals coordinate the aforementioned actions seems to deteriorate during the aging process. In SCI individuals, there is little evidence to show that neither the PNS nor CNS prematurely declines in these people [58].
Neuropathic pain (NP) is common following SCI and can substantially reduce the person’s ability to function and their quality of life. Neuropathic pain typically occurs at the SCI level or below of the lesion being characterized by physical characteristics such as temperature (e.g., hot, burning, sunburned, frostbitten) and electricity (e.g., an electric shock, tingling, stabbing, shooting pain). Pain can be apart from any external stimulus (rest pain) or can result from a stimulus that, under normal conditions, wouldn’t cause pain (allodynia), or pain can be excessive in response to a painful stimulus (hyperalgesia). The NP may be intermittent or constant and may fluctuate in intensity. These symptoms may result from a reactive synaptogenesis changes in central neuronal function, altering thresholds of the pain response. The evaluation of neuropathic pain following SCI requires knowledge about spine biomechanics, spine neurophysiology, and differentiation between mechanical versus neuropathic origin of the pain. The underlying condition many times is not identified but sometimes may be due to syringomyelia, scar tissue, tethering cord, unstable spine, posttraumatic cyst formation, and others. NP may be exacerbated by the weather change and at times by an unrelated disease or medical complications (e.g., renal stone, urinary tract infection, fecaloma). Patients are relieved to be informed that their pain need not reflect any active problem and need not cause them to curtail their activities. Indeed, an increased level of activity may decrease suffering [59]. Neuropathic pain can be exacerbated by many conditions, but there is no evidence to suggest that neuropathic pain increases with age [60]. Early onset of pain in people with SCI is a strong predictor of future pain. Approximately two-thirds of people with SCI experienced some form of pain, with one-third of these reporting severe pain [61]. People with pain related to their SCI are often unable to gain significant relief from pharmacological medications or have to discontinue their use due to side effects [61]. Psychosocial and environmental factors have been shown to play a key part in the experience of chronic pain in people with SCI [62].
Posttraumatic syringomyelia is a progressive enlargement of a cystic cavity (or syrinx), which originates at the site of injury in the spinal cord, and may also occur in people with SCI, causing neurologic deterioration [63]. Onset may take months to years after injury, being heralded by changes in neuropathic pain, or spasticity, deterioration in function with an ascending sensory loss with or without motor weakness, or, sometimes, autonomic symptoms such as increased sweating.
According to Young, spasticity (SPS) is a “velocity dependent increase in muscle tone with exaggerated tendon jerks resulting in hyper-excitability of the stretch reflex” in combination with other features of the upper motor neuron syndrome [43]. Furthermore, Young had divided the characteristic signs of upper motor neuron damage in positive and negative. The positive ones include increase of muscle tone and tendon jerks, clonus, extensor stretch reflexes, and released flexor reflexes such as Babinski reflex. The negative ones are paresis, loss of fine motor control and loss of dexterity, increased fatigability of muscles, and hypotonia in early phase of upper motor neuron damage.
Clinically, spasticity causes resistance to passive motion of the limbs, exaggerated deep tendon reflexes, clonus, and involuntary co-contraction of muscle groups. SPS can follow complete and incomplete SCI. Usually SCI is immediately followed by a period of flaccidity, with spasticity developing over weeks. SPS has favorable effects as well as unfavorable. It can be used to assist with mobility, can improve circulation, and may be useful for decreasing the risk of deep venous thrombosis and osteoporosis. On the other hand, spasticity can interfere with positioning, mobility, and hygiene, and spasms can be painful. When making a decision to intervene, one must take into consideration both the positive and the negative aspects of a patient’s spasticity and the degree and type of the patient’s spasticity.
According to our knowledge, there is no evidence to show the change of the symptoms of spasticity in SCI individuals during the years or a long-term effect of the treatment of spasticity.
Immediately after SCI, the presence of pain appears to be the best predictor of future pain, and this likely does not change significantly over time [64, 65].
In general, there is a continued dearth of knowledge regarding the aging SCI nervous system.
Depression and Cognitive Functioning
In people with SCI, depression affects the subject in different ways. It affects mood, ambition, outlook, problem solving, and energy levels. It works against wellness and health and against having a good quality of life. Individuals with SCI who are depressed often have more difficulty looking after themselves and managing their medical condition; they may have difficulty managing a correct diet, drinking sufficient water, taking care of their skin, taking medications, and posturing correctly on a wheelchair. Although depression is common among people with SCI, many people with SCI never experience an episode of depression. Aging with an SCI/D is more difficult for women. McColl [66] found that age, gender, and disability result in an excess of depression among older women with SCI. Krause [67] and co-workers suggested that minorities in SCI are at greater risk for depression, particularly minority women, but that education and income largely account for the elevated risk. This study also reported that individuals who were older at SCI onset, beginning with the ages of 30–39, were at greater risk for both presence of clinically significant symptoms of depression and major depression. They conclude saying that “symptoms of depression are highly prevalent after SCI and are related to aging, gender or ethnicity, and socioeconomic status indicators (education and income).” The older the individual at SCI, the more dramatic the adaptation required.
In response to aging with SCI, it is difficult to say that those who have lived the longest with SCI have experienced the higher levels of depression or if the onset of depression is consequent to the secondary conditions resulting from aging [8]. In our experience, working with SCI for a long time, like for the general population, behavior and emotion in response to an injury are a complex interaction of own personality, social/family support, level of education, and economic possibilities. In essence, every SCI, and every person with SCI, is different, and the coping mechanism to the SCI seems to depend more to the social support of the individual, level of education, and economic support rather than his or her unique personality or his level of injury; currently, no one can accurately predict who or when one of these individuals will become depressed. Healthcare providers need to look out for the signs and symptoms of psychological stress and provide quality information and support to people in a time-appropriate manner. Recommendations for maintaining psychological health include maintaining social connections and being proactive within the community, doing physical activity, encouraging the uptake of a hobby/activity, and making sure the person with SCI knows when to ask for help, should he need it.
Cognitive impairment following SCI is high and seems to reach 40–50% of SCI [68]. However, such deficits are frequently attributed to concomitant brain injury [69–73] or to premorbid conditions such as poor intellectual or occupational functioning [74], previous brain injuries, alcoholism or drug abuse [69], low blood pressure [75], and psychiatric disorders. Some studies exploring cognitive functioning following SCI in the absence of the abovementioned conditions report deficits in processing speed, learning, memory, and attention [71, 76]. When concomitant head injury is ruled out, one prominent explanation for such deficits involves secondary changes to brain organization or activity resulting from SCI [77]. Reactive depression has also been highlighted as a possible source of cognitive decline [78–80] and negative influence on cognitive performance [81]. Finally, recent research showing changes in spinal excitability [82] and plasticity [83] during learning supports the possibility that decline in certain cognitive functions is linked to spinal cord damage directly. Kowalczyk et al. [84] demonstrated that the alterations found in motor function in cervical myelopathy secondary to degenerative disease and spinal cord compression are due not only to local effects of spinal compression but also to distal effects related to cortical reorganization and decreased N-acetylaspartate/creatine in the motor cortex. This study may shed light about some important changes that can happen with aging with SCI. Myelopathy secondary to degenerative disease in older individuals may well represent a model of aging with SCI. In this sense, changes in brain function that may occur may not only be a fruit of natural aging, but instead SCI and spinal cord compression can influence not only the spinal cord and its functions but also the brain function, determining a change in the reorganization of the cortical motor cortex.
Gastrointestinal Changes
Bowel disturbances such as constipation, distension, abdominal pain, rectal bleeding, hemorrhoids, bowel accidents, and autonomic hyperreflexia occur in 27–62% of SCI patients. With the aging process, the SCI aggravates the dysfunctions of neurological intestine [85].
- (a)
Dysphagia: This is present in 22.5–30% of patients with SCI and is related to age, to the presence of a tracheostomy, to the effects of mechanical ventilation [86], and to cervical spinal surgery (anterior approach). As a result, dysphagia can be the consequence of three pathological causes: (a) direct compression of the aerodigestive tract and associated nerves, as well as local inflammation that leads to mucosal edema, formation of adhesions, fibrosis, and cricopharyngeal spasms; (b) swelling of prevertebral soft tissue, due to repetitive mechanical trauma (i.e., dynamic constant movement of the pharyngo-laryngo-esophageal complex over a rigid structure/bony protuberance), causing a small movement of the pharyngeal wall, altered upper esophageal sphincter opening, incomplete epiglottic deflection, and a vallecular, piriform sinus and posterior pharyngeal wall food residue; and (c) presence of several anatomical structures at risk of damage during the anterior cervical spine surgery. Surgical procedures involving the cervical spine C3 or higher can lead to damages of cranial nerves glossopharyngeal (IX) and hypoglossal (XII), while those made in the C3–4 region endanger the superior laryngeal nerve (SLN).
A deficit in the oral phase of swallowing points out a possible damage of cranial nerves hypoglossal and glossopharyngeal, compromising the propulsive action of the tongue. Weakened pharyngeal swallowing suggests disruption of pharyngeal plexus and the pharyngeal muscles, which can occur with injury to the SLN [87]. The vagus nerve, although normally protected by the carotid sheath during anterior cervical exposures, is vulnerable to retraction injury at any subaxial cervical levels.
- (b)
Pýrōsis or heartburn: This symptom is the result of incomplete upper esophageal sphincter relaxation, while resting upper and lower esophageal sphincter pressures may be normal. Delay with gastric emptying (GE), recumbency position, immobilization, and the use of certain drugs (such as anticholinergics and meperidine) predispose to pýrōsis. Heartburn may predispose some SCI patients to lung aspiration or “ab ingestis” pneumonia. Gastric emptying (GE) times increase directly with the higher level of the SCI and the increased duration of injury. Intravenous metoclopramide, a potent dopamine receptor antagonist with prokinetic properties, corrects impaired GE [88, 89].
- (c)
Fecal impaction: This is a common GI complication in SCI [90, 92]. It is related to reduction of colonic mass movements and inability to use abdominal muscles to assist in defecation. Inadequate dietary fiber and water intake is the primary cause, but lack of mobility and the inability to use abdominal muscles in the aging population of SCI patients can predispose to this disorder more often. Constipation in complete SCI may also be related to previous urinary outlet surgery and the use of anticholinergic agents to manage the neurogenic bladder [88].
Patients may present with loss of appetite and nausea and are mistakenly given antinausea preparations, most of which have anticholinergic and constipating properties. Liquid stool may pass around the blockage. Plain films of the abdomen show the feces and abnormal air patterns. Treatment involves digital disimpaction and proximal or distal washout and anal fragmentation of the hardened stool palpable in the rectum. At times manual disimpaction may be aided by the use of an anal retractor.
Oral solution of polyethylene glycol (PEG) solutions is used to soften or wash out proximal stool. Oral regimens vary from 1 to 2 L of PEG with electrolytes or 17 g of PEG 3350 in 120 to 240 mL of water every 15 min until the patient begins passing stool or eight glasses have been consumed [91]. This technique is contraindicated when a bowel obstruction exists.
Enemas and suppositories are used as distal softening agents. Most enema solutions contain water and an osmotic agent (one of such combinations contains water, docusate sodium syrup, and sorbitol). When the impaction has been adequately treated, possible etiologies are explored. A total colonic evaluation (colonoscopy or barium enema) should be performed to reveal anatomic abnormalities (stricture or malignancy). Endocrine and metabolic screening, including thyroid function tests, is also indicated [92].
When the abovementioned methods of fecal evacuation have failed, cisapride, a 5-HT4 serotoninergic agonist drug facilitating the release of acetylcholine at the myenteric plexus, can be used. Cisapride is able to increase gastrointestinal motility especially in the small intestine and colon, but one of its most feared adverse effects is the appearance of a long QT syndrome that can predispose the individual to torsades de pointes and often fatal arrhythmia (for that reason it has been taken off the market in the USA).
Neostigmine is a drug, which increases cholinergic tone by blocking the metabolism of acetylcholine by acetylcholinesterase and can be effective in the treatment of constipation. It was tested in combination with glycopyrrolate (an anticholinergic agent that reduces bradycardia and bronchoconstriction caused by neostigmine) [93].
- (d)
Megacolon: Although SCI age over 50 years presented an almost threefold increase in risk for the presence of megacolon, the origin of megacolon is not well understood. Two hypotheses seem to support the origin; the first is the acquired origin, and the second is the level of spinal cord injury.
In supporting the acquired origin hypothesis of megacolon, Harari and Minaker reported that, in the first 5 years of SCI, it is rare for the phenomenon of constipation to occur and a post-injury period of 10 years or more placed patients at an almost fourfold greater risk of having megacolon. This finding supports the abovementioned hypothesis, stating that the problem is acquired, possibly through degeneration or decompensation of smooth muscle in the intestine [94]. Following the paralysis of the peristaltic movements of the intestine, the colon loses its shape acquiring an abnormal dilated form. Distressing symptoms such as prolonged and difficult evacuation, recurrent abdominal distension, and abdominal pain tend to develop, and standard treatments for constipation become less effective. As the use of laxatives and suppositories increases, so does the incidence of bowel bloating, nausea, and fecal impaction, and as a consequence, the megacolon develops.
The second hypothesis finds its support studying the chronic SCI; some researchers believe that most likely the pathophysiological events responsible for the abnormal dilatation of the colon depend from the level of SCI [90, 94, 95].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree