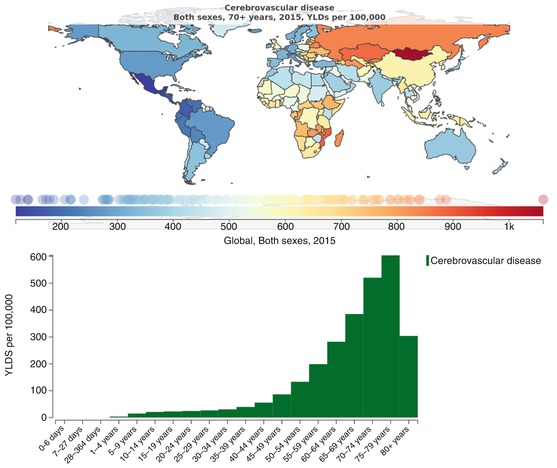
Fig. 32.1
YLDs (years lived with disability) related to cerebrovascular disease in elderly people in the world and YLDs per 100,000 persons related to neurological condition based on age. Source: https://vizhub.healthdata.org/gbd-compare/
32.2 Brain Reserve and Neuroplasticity
Brain reserve is the brain’s resilience to pathological damage or changes. The greater the brain reserve, the less likely an individual will demonstrate disturbance associated with a central nervous system (CNS) lesion. The majority of studies focused on the concept of cognitive brain reserve, confirming that the level of education and cognitive skills in general (i.e., bilingualism, multiple hobbies, etc.) retard the onset of dementia or cognitive impairment [12, 13]. An emerging concept is motor brain reserve, in which neurophysiological/neuroimaging or structural markers are searched to predict who will recover better from a motor perspective after a CNS insult.
Motor reserve has been linked to the strength or modularity of cerebral motor networks: the better cortical areas involved in motor behavior are interconnected and maintain communication after the insult, the better the motor recovery will be. These assessments, conducted either with high-density EEG or with functional resonance imaging (fMRI), are time-consuming and require highly skilled teams. An alternative surrogate has been suggested to be cortical thickness of motor areas, or the extent of the cortex covered by the hand knob: both parameters require a simple structural MRI and can be determined by ad hoc software [14].
The issue of brain reserve parallels that of neuroplasticity, in that a higher functioning brain is likely to have higher potential for neuroplasticity. Neuroplasticity is the potential of the brain to rearrange and rewire; it can be part of a physiological process, i.e., during growth, or a reaction to a damage to the CNS. It affects microscopic level, e.g., by modifying neurotransmitters release or modulating synaptic expression, mesoscopic level with modifications of neuronal ensembles interactions, and macroscopic level when the cortex rearranges and modifies its structure.
We refer the reader to Chap. 2 (the ageing brain) for a more detailed discussion of neuroplasticity in the elderly.
32.3 Rehabilitation of Functional Impairments
According the World Health Organization (WHO), the aim of rehabilitation is to maximize function and minimize limitation of activity and restriction of participation resulting from an underlying impairment or disease, promoting an innovative paradigm in rehabilitation. WHO’s International Classification of Functioning, Disability, and Health (ICF) includes medical, biological, and social aspects of disability, and can be used as a framework to collate all elements and goals in rehabilitation.
Rehabilitation should boost residual functions, and not focus on lost capacities. It might not be possible to return to motor baseline performance, but personal and psychological resources and remaining functions shall be the basis for a sound rehabilitation.
Rehabilitation typically entails a cyclical process [15], involving four steps as detailed in Table 32.2.
Table 32.2
Four steps in the rehabilitation process
1 | Assessment | To identify and quantify the person’s needs |
2 | Goal setting | To define realistic and attainable goals for improvement |
3 | Intervention | To assist in the achievement of goals |
4 | Reassessment | To assess progress against agreed goals |
Healthcare professionals working in neurological rehabilitation need to explore the social, psychological, and cultural background of the person to be rehabilitated, to identify expectations and values, and agree upon goals with each individual during the entire rehabilitation process. An increasing literature is highlighting the advantages provided by self-management programs [16], which include, among other interventions, involving people in decision making, emphasizing problem solving, promoting healthy lifestyle, and educating people on how to self-manage. Setting agreed-upon plans and following up on the extent to which goals are achieved have been reported the most effective intervention [17] in these programs, stressing the need for appropriate goal setting paradigms [18].
Given the complexity of impairments of elderly people with neurological dysfunctions, the need for multidisciplinary teams has been advocated. The integrated competences of neurologists, geriatricians, physical medicine doctors, physical and occupational therapists, neuropsychologists, speech and language therapists, and social workers are highly recommended.
32.3.1 Clinical Presentation and Functional Impairments in Stroke, Traumatic Brain Injury, and Multiple Sclerosis
32.3.1.1 Stroke
Stroke is the consequence of impaired oxygen supply to a brain region. It can be ischemic if the defective supply depends on either a vessel occlusion by an embolus or a thickening of the arterial wall or reduced perfusion, or hemorrhagic if a ruptured vessel impairs cerebral blood flow. In the latter case, the detrimental effects of hypoxia are coupled with the irritative effects of hemoglobin derivatives on brain tissue. Clinical presentation is heterogeneous, according to the lesioned brain area or to the disrupted cerebral network. Motor and language impairment are frequent signs following a middle cerebral artery occlusion, but symptoms range from sensory and visual disturbances to full-blown neuropsychological deficits. Due to the phenomenon of diaschisis (Greek for disconnection), some deficits regress spontaneously during the first days post-stroke, as the neural connections between distant areas are restored or alternative ones created.
Stroke treatment has advanced significantly over the recent years. The concept of ischemic penumbra, i.e., the tissue surrounding the ischemic core and still viable, which could be rescued if an adequate blood flow can be reinstated, has provided the conceptual basis for a paradigm shift in stroke therapy. Evidence of the benefits of thrombolysis and intra-arterial interventions, effectiveness of the multidisciplinary stroke unit [19], as well as the focus on rapid transfer of people with stroke to hospital has transformed the treatment. This change saves lives and improves outcome. However, only a small proportion of the stroke population will benefit from thrombolysis or mechanical clot retrieval. Stroke survivors will mostly still be in need of rehabilitation provided by the multidisciplinary team starting from the stroke unit up to the community level.
Many stroke survivors are left with significant residual disabilities, with 22% unable to walk again and between 24% and 53% requiring assistance with activities of daily living [20–22]. Functional outcome has been related to clinical signs [trunk control and lower-limb (LL) strength i] [23], integrity of corticospinal tracts [24, 25], and neuronal reorganization and cortical connectivity [26–28].
32.3.1.2 Traumatic Brain Injury
Traumatic brain injury (TBI) refers to damage to the brain caused by an external physical force. The leading cause of brain injury in the elderly is falls [2]. Older age, comorbidity, and medications including blood thinners are all risk factors for increased severity. CT scans in the acute setting and after a few months from the event to exclude subdural hematomas are recommended as part of the diagnostic process. Injuries that require admission to a neuro-intensive care unit usually undergo the same functional assessment as stroke patients.
In a closed head injury, damage occurs because of a blow to the person’s head or having the head stop suddenly after moving at high speed. This causes the brain to move forward and back or from side to side, such that it colliding with bony structures that surrond it. This jarring movement bruises brain tissue, damages axons, and tears blood vessels. Edema often ensues, increasing intracranial pressure, which can cause further damage to the brain by preventing blood flow to the tissue. Therefore, after a closed head injury, damage can occur in specific brain areas (localized injury) or throughout the brain (diffuse axonal injury). Instrumental investigations assessing damage severity include neurophysiological recordings [electroencephalogram (EEG); somatosensory evoked potentials (SSEPs)], neuroimaging with diffusion tensor imaging (DTI) and diffusion weighted imaging (DWI), and measurements of increased intracranial pressure.
The goal of the acute treatment is to prevent any further, or secondary, injury to the brain. Decreasing and controlling intracranial pressure is a major target of medical treatment in the early phase after trauma. According to the severity of TBI and the residual damage, the issues posed to the rehabilitation physician vary. More mild cases can present with little or no motor impairment, but present a variety of cognitive impairments, for which cognitive and vocational, if the person is still in working age, rehabilitation are advised. More severe cases usually present with motor and sensory deficits and spasticity, the degree of which is often important and requires drastic medical therapies, and cognitive disturbances that can include persistent alterations of consciousness. A comprehensive approach to patient and family is required in these cases, offering an adequate psychological support to family members.
32.3.1.3 Multiple Sclerosis
Multiple sclerosis (MS) is an inflammatory disease of the central nervous system. Typically, MS is diagnosed between age 20 and 50. Its course varies widely, with a relatively benign relapsing–remitting form with a restitution ad integrum in between attacks, to aggressive primary progressive forms which cause severe disability early on in the course of the disease. Life expectancy is only slightly reduced due to MS (6–7 years reduction in a large French cohort) [29]. Older people with MS will undergo normal age-related changes in function as well as those associated with MS progression. Involvement of any site in CNS is possible; this implies a variety of sign and symptom, which range to motor and sensory impairments, cerebellar dysfunctions, sphynteric problems, dysphagia. Cognitive deficits develop later in the course of the disease. Functional impairments thus vary according to the neurological signs and the severity of disease progression. There is a disability progression with disease duration, with the percentage of subjects in need of walking aids increasing from 15% to 76% after an average 45 years of disease, and a 63% of subjects independent in activities of daily living at disease onset compared with a mere 8% in older age [30]. Progression rates have been substantially lowered after the advent of disease-modifying therapies [31].
32.3.2 Rehabilitative Approaches to Impairments Due to Lesions of Central Nervous System
Neurological rehabilitation foundation rests on the concepts of restoration and compensation.
In the very early phases after an acute event, restoration is more likely to happen [32]. Restoration is based on the potential of brain tissue adjacent and/or distant from a lesion to undergo plastic changes that include axonal sprouting, rewiring of previously unconnected brain areas, and modulation of cerebral excitation/inhibition to promote behavior. These changes can be facilitated, in motor deficits, by repetitive task-specific training, with high numbers of repetitions providing the strongest stimulus for neuronal rearrangement. Excessive or non-correctly directed training can indeed have detrimental effects on plasticity, possibly facilitating the so-called maladaptive plasticity—i.e., changes in brain function that foster dysfunctional interactions of cortical areas that translate in defective behavior.
Conversely, compensation is based on the incorporation of residual competencies into functional motor schemes, in order to provide the subject with the means of performing a task in alternative ways—may it be using a different motor strategy or a device.
32.3.2.1 Stroke
After discharge from the acute stroke unit, standardized pathways guide the rehabilitation process, stressing the need for multidisciplinary care, optimizing length of stay in intensive neurorehabilitation units, and providing a framework for early supported discharge. Early supported discharge is an approach that promotes discharge from hospital to community-based rehabilitation as soon as possible once appropriate support is in place for both patient and carer.
Evidence is building up that it is quantity of rehabilitation to impact on outcome [33, 34], with task oriented training particularly effective [35]. One study was able to provide up to 300 h of therapy over 5 weeks [33] compared to the standard-of-care 75 h over the same period: the authors reported an increase of the Fugl-Meyer score between 8 and 11 points.
Short and frequent early rehabilitation sessions have been demonstrated to improve the odds of favorable motor outcome after 3 months from the event [36, 37]. Involvement of family members in an early stage can partially replace resources and help for rehabilitation needs.
Interventions seek to restore motor control in gait and transfer, to improve upper limb activities, to assist in coping with activities of daily living (ADL), and to enhance participation. Advice and instructions are provided to the subject, family, and other members of the interdisciplinary stroke team regarding treatment and prevention of complications such as shoulder pain, venous thrombosis, and falls.
The optimal time window for physical activity after stroke, the intensity, type of activity, and duration for best brain repair processes are not yet fully elucidated; indeed, we know that a neuro-restorative window closes after 3 months from the event [32] and that excessive physical strain in the very early phases of recovery can be counterproductive [38]. However, moderate forced exercise seems to reduce lesion volume and protect perilesional tissue against further oxidative damage and inflammation at least for the short term (4 weeks) in post-stroke patients [39].
Different treatment approaches have been tested to improve function.
A recent Cochrane review [40] reported moderate-quality evidence of a beneficial effectfor upper limb functional recovery of constraint-induced movement therapy (CIMT), mental practice, mirror therapy, interventions for sensory impairments, virtual reality, and a relatively high dose of repetitive task practice, suggesting that these may be effective interventions. Moderate-quality evidence also suggested that unilateral arm training may be more effective than bilateral arm training. The same authors provide additional guidance on fields still to explore, such as non-invasive brain stimulations, hands-on therapy, music therapy, pharmacological interventions; they also provide updated reviews on the efficacy of biofeedback, Bobath therapy, electrical stimulation, reach-to-grasp exercise, repetitive task training, strength training, and stretching and positioning.
For upper limb rehabilitation, constraint-induced movement therapy (CIMT), possibly associated with robotics and mental practice with motor imagery, was shown to be beneficial in improving arm function. CIMT consists in the forced non-use of the healthy arm, on which a mitten is worn, in order to force the use of the affected side. Subjects who benefit more from CIMT are those with active wrist and finger extension on the affected side. However, open questions remain on the applicability of CIMT, ranging from the number of hours needed per day, that in the majority of studies is very high, to its long-term effects, to the detrimental psychological consequences in severely impaired subjects that end up frustrated by the little functional use of the affected hand whilst having the healthy one blocked.
Mirror therapy builds on the concept of mirror neurons, i.e., neuronal ensembles that fire when a person observes an individual of a similar species performing a motor task [41]. In clinical practice this has been translated in exercises performed with the healthy limb using a box with inbuilt mirrors in which the affected arm is positioned: the final effect is that the subject observes his/her healthy limb moving in the mirror, as if observing the affected arm instead, thus promoting the functioning of mirror neurons and the rearrangement of sensory motor areas (SM). Motor imagery rests on the same neurophysiological principle: Rearrangement of SM by motor imagery is supported by neurophysiology and neuroimaging, which provides evidence of an overlapping activation of sensorimotor cortices with active, passive, or imagined movements [42–44]. Mentally rehearsing a movement thus promotes the same mechanism as active movement, and is indeed a technique used also by professional musicians and sport players.
Interventions for sensory impairment vary widely—e.g., stimulation with different surfaces, modulation of limb positioning in space, etc.,—and are all based on the recovery of the proprioceptive incoming information on which movement is tuned or the recovery of afferent inputs to central sensory areas.
Robotic devices are getting momentum in rehabilitation, given the chance to provide high-intensity, repetitive, task-specific, interactive treatment of the impaired limb (passive and/or active-assisted exercises). In addition, they allow monitoring subjects’ motor recovery, measuring changes in forces and movement kinematics. These robotic devices demonstrate improvement of motor function and, in few studies, also of strength of the upper limb, but generally not of performances in ADLs, raising the question of the generalizability of this training. For the moment being, no guidelines on UL robotic training exist; a recent revision [45] shows that robotic treatment cannot be considered a stand-alone approach, but needs to be integrated with conventional therapy. In addition, often elederly subjcets are less familiar with such devices and thus less likely to benefit of this training.
Simultaneous bilateral training may be no more effective than other upper limb interventions to improve performance in activities of daily living (ADL) or motor functional outcome of the upper limb, but the methodological quality of studies focusing on this topic may be questioned. Other authors reported better functional outcome after bilateral training based on the differential functional lateralization of upper limb [46].
Physiotherapy is effective in the recovery of lower-limb motor function, postural control, and walking ability especially in the early phase after stroke. The association of electromechanical assisted training for walking has been shown to improve the odds of independent over-ground walking, although only people in the acute stage who are not ambulant seem to show improvement [47].
Interventions to facilitate sitting and standing balance include repetitive task-specific training and biofeedback with a moving platform. Task-specific training might improve sit-to-stand function and standing balance, while biofeedback with a force plate or a moving platform showed improvement in stand symmetry alone. Unequivocal indication on the best approach to facilitate the recovery of balance following stroke is still missing.
Cardiorespiratory physical fitness training is the only interventions with a robust evidence for a benefit on walking ability measured in terms of gait speed [48]; improvements have been obtained also with high-intensity physiotherapy, repetitive task training, and electromechanical gait training with exoskeleton robots or robotic end-effectors. Treadmill walking with body weight support also seems to achieve a better and earlier walking independence after stroke.
The use of virtual reality, a relatively recent approach that simulates the practice of functional tasks, is not yet commonplace in clinical rehabilitation settings, but seems to be effective if coupled with standard therapy [49].
Ankle-foot orthoses might also improve gait performance and reduce energy expenditure of gait in patients who have persistent foot drop.
The aforementioned interventions are generally possible if the subject has a preserved range of motion (ROM), whereas strength is not a prerequisite. The main cause for ROMs reduction is spasticity, a velocity dependent increase of muscle tone that can lead in the long term to rheological modifications and contractures of soft tissues. Pharmacological interventions include oral antispastic agents (baclofen, benzodiazepines, dantrolene, α2 receptor agonists) and injections of botulinum toxin in affected muscles in cases of focal spasticity, or nerve blocks. The use of splinting after treatment is usually recommended.
32.3.2.2 Traumatic Brain Injury
The rehabilitation of subjects with traumatic brain injury is normally distinguished into two phases, the acute and the post-acute phase.
Individuals with TBI are typically admitted to the intensive care unit for close observation and medical interventions.
Some preventive rehabilitation procedures may be initiated in the intensive care unit such as body positioning and early mobilization to prevent secondary damage such as pneumonia, contractures, or pressure decubitus ulcers.
As early as possible, individuals with brain injuries will begin intensive rehabilitation.
The focus is on retraining activities of daily living, pain management, cognitive and behavioral therapies, pharmacological management of medical issues or of spasticity, with the option of administration of intrathecal baclofen via an implantable pump, assistive technology (e.g., prescription of wheelchairs and gait aids), environmental manipulation (e.g., installation of lifts, ramps and rails, and bathroom adaptations), as well as family education and counselling.
Community rehabilitation follows discharge from an inpatient rehabilitation service. Helping a person with TBI return to maximum independence and participation in the community is an extremely difficult task. Family support, education, and counselling are vital and likely to be needed for a prolonged period.
32.3.2.3 Multiple Sclerosis
Although rehabilitation has no direct influence on disease progression, it has been shown to ease the symptoms of MS by enhancing self-performance and independence. Whatever premorbid status, an adapted early mobilization is important to avoid further loss of function. The setup of community services is of uttermost importance in this disease, given the long-term disability it entails.
Physical therapy for MS might includes a physical exercise program, motor and sensory balance training, gait training and training in the use of mobility aids (canes, crutches, wheelchairs), and other assistive devices. Aerobic training can help to enhance aerobic capacity and isometric strength, and possibly ease psychological disturbances (anxiety and depression) and fatigue. Respiratory training can be effective for improving respiratory functions and cough reflex. Physical therapy can also include pelvic floor training which may help address urinary/bladder symptoms (incontinence, urgency, and frequency).
Spasticity can initially be managed with changes in daily activities or physiotherapy. If these approaches are unsuccessful, pharmacological treatments can be beneficial in the management of spasticity. Pharmacological management should always be accompanied by physiotherapy.
Training in swallowing with triggering of reflexes, training of the swallowing process, compensatory measures, and appropriate consistency of food and liquids can help to improve the process of swallowing and reduce the risk of aspiration.
Occupational therapy provides training in energy conservation techniques and the use of adaptive tools and devices to simplify tasks at home and at work. Strategic modifications to the home and workplace are recommended to ensure accessibility.
32.3.3 Clinical Presentation and Functional Impairments in Neuropathies
Neuropathy is a disorder of peripheral nerves, characterized by a damage to the myelin sheet or the axon, which causes altered conduction in sensory or motor nerves. Typical symptoms can be pain, paresthesia, dysesthesia, and/or hypostenia. More severe forms include vegetative symptoms and/or trophic changes. More than 100 forms have been described [50]. Frequent forms in the older are chronic demyleinating neuropathies, often associated with hematological alterations. Motor and sensory symptoms can both be present. Some forms are paraneoplastic (i.e., caused by a likely cross-reaction with tumor antigens) and are expected to progress in parallel with the underlying disease. Small fibers neuropathies are also common in older age and cause burning pain and unpleasant temperature sensation.
Among the most disabling neuropathies are the acute inflammatory forms, which are clinically and pathophysiologically heterogeneous group. Guillain–Barré syndrome (GBS) is an acute polyneuropathy consisting of different subtypes. In as many as two-thirds of GBS patients, the onset of weakness occurs 1–3 weeks following an upper respiratory illness, gastrointestinal infection, or vaccination. Incidence is 1.1 cases per 100,000 people per year in the northern hemisphere [51]. Therapy includes plasma exchange and intravenous immunoglobulin. The typical clinical picture is one of diffuse, progressive weakness, which can in the most severe cases involve also respiratory muscle. Sensory involvement is exceptional.
GBS and other inflammatory polyneuropathies are a group of disorders that is often associated with significant long-term disability. In addition to motor or respiratory deficits, affected people develop psychosocial problems resulting in complex disability, which may require treatment in a specialist rehabilitation service. However, in comparison to other long-term neurological conditions (such as brain injury, stroke, or multiple sclerosis) there are relatively few published studies of rehabilitative treatments and outcome. Further research is needed to define treatments to prevent 20% of subjects from being left with persistent and significant disability. Guillain–Barré syndrome is thought to be amendable to multidisciplinary care, but the evidence base for its effectiveness is unclear [52].
32.3.3.1 Rehabilitative Approach to Hypostenia of Peripheral Origin
The prolonged immobility experienced by many patients with hypostnia puts them at significant risk for position-related nerve compression, skin ulceration, and contractures. Subjects that are intubated/sedated and those with significant sensory loss may not notice the symptoms that typically occur with these injuries. Careful body positioning, appropriate bracing, pressure point padding, and frequent position changes are all warranted. People with incomplete eye closure from facial weakness are also at risk for exposure keratitis. Good corneal hygiene with artificial tears, lubricants, careful lid-taping, or protective eye domes is essential [53].
Physical therapy should be initiated as soon as possible. In severely affected people, passive range-of-motion exercises prevent contractures. As subjects improve, other modalities and functional exercise programs are recommended. Despite scarce evidence-based data, physical therapy for GBS as an inpatient and continuing upon discharge is associated with better outcomes and recommended for all but the mildest cases. Physical therapeutic modalities involve a progressive mobility program, encompassing maintenance of posture, alignment, and joint range of motion, provision of orthotics, endurance exercises, muscle strengthening, and gradual gait training using gait aids. Care should be paid not to overwork muscle groups; exercise programs should initially be non-fatiguing and targeting antigravity muscles; progressively, more aggressive strengthening exercise can be prescribed. Overworking muscles in people with peripheral nerve involvement can lead to paradoxical weakening. Stretching program can prevent the onset of muscle contractures.
Muscle weakness, paralysis, balance impairment, and fatigue may result in need of assistive devices. The option for mobility devices varies from ankle-foot orthoses, canes, crutches, walkers, and wheelchairs. People with prolonged residual weakness of calf, and most commonly anterior compartment musculature, benefit from ankle-foot orthosis and shoes with good stabilization around the ankle joint.
32.3.3.2 Rehabilitative Approach to Hypoesthesia, Pain, and Proprioceptive Deficits of Peripheral Origin
Hypoesthesia rehabilitation after a peripheral nerve lesion is based on plastic reorganization of the somatosensory cortex: training aims at eliciting function of the residual sensory nerve fibers, whichever type they may be, and associate the re-learned sensation. Programs include touch exercise, in which the subject touches different objects/surfaces comparing them with the unaffected limb with a growing level of complexity, transcutaneous vibratory stimulation and global complex activities in which motor and sensory tasks are combined.
Pain is a common but often overlooked feature of neuropathies, occurring in more than half of the patients subjcets. It often precedes the development of weakness due to the higher vulnerability of sensory nerve fibers and persists long after recovery [53]. Transcutaneous electrical nerve stimulation (TENS) may be effective in reducing pain, but there are diversities in published research regarding its effectiveness and results are not conclusive. Pharmacological therapy can be an option.
Chronic demyelinating polyneuropathies have recently [54] shown to be sensitive to proprioceptive perturbation despite sensory impairment, encouraging a rehabilitation aimed at promoting the recovery of proprioceptive information rather than the compensation by visual information.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree