Platelet function tests have been traditionally used to aid in the diagnosis and management of patients with bleeding problems. Given the role of platelets in atherothrombosis, several dedicated platelet function instruments are now available that are simple to use and can be used as point-of-care assays. These can provide rapid assessment of platelet function within whole blood without the requirement of sample processing. Some tests can be used to monitor antiplatelet therapy and assess risk of bleeding and thrombosis, although current guidelines advise against this. This article discusses the potential utility of tests/instruments that are available.
Key points
- •
Platelet function testing immediately presents a series of unique problems to any laboratory or clinic. Platelet function testing assesses the dynamics of living cells in contrast to tests that determine a defined quantity or measurement of a clinical biomarker (eg, cholesterol or blood pressure).
- •
Most platelet function tests have traditionally been used for the diagnosis and management of patients presenting with bleeding problems rather than thrombosis.
- •
Platelet function testing is poorly standardized and normal quality control measures are often difficult to implement.
- •
Platelet function tests are increasingly used for monitoring the efficacy of antiplatelet drugs, with the aim of predicting bleeding or thrombosis.
- •
Current guidelines advise against routine monitoring of antiplatelet therapy with platelet function tests.
Introduction
It is becoming increasingly clear that platelets are multitalented, with many recently discovered new functions (inflammation, host defense, fetal vascular remodeling, tumor growth and metastasis, and so forth). Nevertheless, the best established role of platelets remains that of maintaining normal hemostasis, with any imbalance resulting in pathologic bleeding or thrombosis. Most platelet function tests have traditionally been used for the diagnosis and management of patients presenting with bleeding problems rather than thrombosis. Because platelets are implicated in the development of atherothrombosis, however, which is responsible for considerable mortality and morbidity in the Western world, new and existing platelet function tests are increasingly used for monitoring the efficacy of antiplatelet drugs to treat these conditions and/or to try to identify patients at risk of arterial disease. Conversely, as increasing numbers of patients are being treated with antiplatelet drugs, there is an associated increased risk of bleeding, especially during trauma and surgical procedures. Platelet function tests are, therefore, also increasingly proposed as presurgical/perioperative tools to aid in the prediction of bleeding and for monitoring the efficacy of various types of prohemostatic therapies. The enhanced clinical need coupled with the development of new, simpler tests and point-of-care (POC) instruments, has resulted in an increasing tendency of platelet function testing to be performed away from specialized clinical or research hemostasis laboratories, where the more traditional and complex tests are still performed.
This article discusses currently available clinical tests of platelet function. Although some assays study global hemostasis as a whole, most platelet function assays target a specific phase of platelet function. Table 1 provides a detailed summary of currently available platelet function and hemostatic tests that depend to some extent on platelets, coupled with their clinical utility, advantages, and disadvantages.
| Name of Test | Principle | Advantages | Disadvantages | Frequency of Use | Clinical Applications |
|---|---|---|---|---|---|
| Adenine nucleotides | Measurement of total and released nucleotides by HPLC or luminescence | Sensitive | Sample preparation, assay calibration, extra equipment | Widely used in specialized laboratories, usually in conjunction with LTA | Diagnosis of storage and release defects |
| AspirinWorks | Immunoassay of urinary 11-dehydrothromboxane B 2 | Measures stable thromboxane metabolite, dependent on COX-1 activity | Indirect assay, not platelet specific, renal function dependent | Increasing use | Monitoring aspirin therapy and identifying poor responders at increased risk of thrombosis |
| Bleeding time | In vivo cessation of blood flow | In vivo test, physiologic POC | Insensitive, invasive, scarring, high CV | Was widely used, now less popular | Screening test |
| Blood smear | Microscopic analysis of blood cell on glass slide | Diagnostic | Artifacts can occur | Widely used | Detection of abnormalities in platelet size, number and granules, and leukocyte inclusion bodies |
| Clot retraction | Measures platelet interaction with fibrin | Simple | Nonspecific | Not widely used | Detection of abnormalities in integrin αIIbβ3 and fibrinogen |
| Combined aggregometry and luminescence | Combined WBA or LTA and nucleotide release | Monitors release reaction with secondary aggregation | Semiquantitative | Widely used in specialized laboratories, although less than LTA | Diagnosis of a wide variety of acquired and inherited platelet defects, diagnosis of storage and release defects |
| Electron microscopy | Ultrastructural analysis of platelets | Diagnostic Whole mount technique useful for dense granular imaging | Expensive, specialized equipment | Only available in special units | Detection of granular and ultrastructural defects, whole mount method can detect dense granular defects |
| Flow cytometry | Measurement of platelet glycoproteins and activation markers by fluorescence | Whole blood test, small blood volumes, wide variety of tests | Specialized operator, expensive, samples prone to artifact unless carefully prepared | Widely used | Diagnosis of platelet glycoprotein defects, quantification of glycoproteins, detection of platelet activation in vivo or in response to agonists, monitoring antiplatelet therapy (eg, VASP phosphorylation to monitor P2Y 12 inhibition) |
| Full blood count | Automated impedance and/or flow cytometry–based analysis of cells | Rapid, precise, provides platelet distribution and MPV | Less accuracy and precision at <20 × 10 9 /L | Widely used | Abnormalities in platelet number, size, and distribution; immature platelet fraction now available |
| Ichor Plateletworks | Platelet counting preactivation and postactivation | Rapid, simple, POC, small blood volume | Indirect test measuring count after aggregation | Used in surgery and cardiology | Monitoring antiplatelet drugs, prediction of bleeding |
| Impact cone and plate(let) analyzer | Quantification of high shear platelet adhesion/aggregation onto surface | Small blood volume required, high shear, rapid, simple, research (variable) and fixed versions available POC | Dependent on fibrinogen and VWF for adhesion of platelets | Mainly research but increasing use | Detection of inherited and acquired defects in primary hemostasis, detection of platelet hyperfunction, monitoring antiplatelet therapy |
| Kinetic aggregometer | Monitoring fluorescent platelet accumulation within a thrombus forming in a collagen-coated capillary | Sensitive to amplification pathways | Specialized laboratories only | Mainly Research | Monitoring antiplatelet therapy, detection of defects in primary hemostasis |
| Laser platelet aggregometer (PA-200) | Monitoring aggregation using a laser | Detection of microaggregates, sensitive | Little widespread experience | Little widespread experience | Detection of platelet hyperfunction |
| LTA | Low shear platelet-to-platelet aggregation in response to classic agonists | Gold standard | Time-consuming, sample preparation, poorly standardized | Widely used in specialized laboratories | Diagnosis of a wide variety of acquired and inherited platelet defects |
| 96 Well plate LTA (Optimul assay) | Similar to LTA but in 96 well plate | Lower blood/PRP volumes than LTA. Many replicates and dose response curves possible | Little widespread experience | Little widespread experience | Detection of defects in primary hemostasis |
| Microfluidic devices | Miniaturized multichannel devices | Whole blood, real-time thrombus formation | Little widespread experience | Research only at present | Detection of defects in primary hemostasis |
| PFA-100/200 | High shear platelet adhesion and aggregation during formation of a platelet plug | Whole blood test, high shear, small blood volumes, simple, rapid, POC | Inflexible, VWF-dependent, Hct-dependent, insensitive to clopidogrel | Widely used | Detection of inherited and acquired defects in primary hemostasis, monitoring aspirin, monitoring DDAVP therapy |
| PlaCor PRT | High shear thrombus formation in duplicate channels Blood oscillates through a narrowed channel with a spring to hold the thrombus | POC; finger prick blood | Little widespread experience | Little widespread experience | Rapid detection of SNPs and gene defects |
| Platelet genome or transcriptome analysis | Total mRNA content by microarray technology | Detection of all mRNAs in platelets and MKs | Instability of mRNA, possible impurity of starting material | Research only at present | Detection of novel gene mutations or transcription defects |
| Proteomic analysis | Measures total individual protein content | Sensitivity increasing | Loss of some membrane glycoproteins, possible impurity of starting material | Research only at present | Detection of protein deficiencies |
| Serum thromboxane B 2 | Immunoassay | Dependent on COX-1 activity | Prone to artifact, not platelet specific | Widespread use | Monitoring aspirin therapy, detection of thromboxane production defects |
| Soluble platelet release markers and sheddome (eg, PF4, βTG, sCD40L, sCD62P, GPV and GPVI) | Usually by ELISA | Relatively simple | Prone to artifact during blood collection and processing | Fairly widely used in research | Detection of in vivo platelet activation |
| Sonoclot analyzer | Impedance detection of changes of viscoelastic properties of celite-activated blood sample | Global test, POC, platelet count and function dependent | Measures clot properties only | Widely used in surgery and anesthesiology | Prediction of surgical bleeding, aid to blood product usage |
| Thromboelastography (TEG or ROTEM) | Monitoring rate and quality of clot formation | Global whole blood test, POC | Measures clot properties only, largely platelet independent unless platelet activators are used | Widely used in surgery and anesthesiology | Prediction of surgical bleeding, aid to blood product usage, monitoring rFVIIa therapy, new platelet mapping system can be used to monitor antiplatelet therapy (eg, aspirin or clopidogrel) |
| ThromboLUX | Dynamic light scattering of platelet quality | Simple, measures size and light intensity of platelets and microvesicles | Designed for platelet concentrates only | Little Widespread experience | Assessment of platelet concentrates |
| VASP | Flow cytometric measurement of vasodilator stimulated phosphoprotein | Measurement of P2Y 12 occupancy | Requires flow cytometer Insensitive to intermediate inhibition of P2Y 12 | Increasing use | Monitoring P2Y 12 receptor activity |
| VerifyNow | Fully automated platelet aggregometer to measure antiplatelet therapy | Simple, POC, 3 test cartridges (aspirin, P2Y 12 , and GP IIb-IIIa) | Inflexible, cartridges can only be used for single purpose | Increasing use | Monitoring antiplatelet therapy |
| WBA | Monitors changes in impedance in response to classic agonists | Whole blood test, multichannel version available. | Older instruments require electrodes to be cleaned and recycled | Widely used in specialized laboratories, although less than LTA | Diagnosis of a wide variety of acquired and inherited platelet defects |
Introduction
It is becoming increasingly clear that platelets are multitalented, with many recently discovered new functions (inflammation, host defense, fetal vascular remodeling, tumor growth and metastasis, and so forth). Nevertheless, the best established role of platelets remains that of maintaining normal hemostasis, with any imbalance resulting in pathologic bleeding or thrombosis. Most platelet function tests have traditionally been used for the diagnosis and management of patients presenting with bleeding problems rather than thrombosis. Because platelets are implicated in the development of atherothrombosis, however, which is responsible for considerable mortality and morbidity in the Western world, new and existing platelet function tests are increasingly used for monitoring the efficacy of antiplatelet drugs to treat these conditions and/or to try to identify patients at risk of arterial disease. Conversely, as increasing numbers of patients are being treated with antiplatelet drugs, there is an associated increased risk of bleeding, especially during trauma and surgical procedures. Platelet function tests are, therefore, also increasingly proposed as presurgical/perioperative tools to aid in the prediction of bleeding and for monitoring the efficacy of various types of prohemostatic therapies. The enhanced clinical need coupled with the development of new, simpler tests and point-of-care (POC) instruments, has resulted in an increasing tendency of platelet function testing to be performed away from specialized clinical or research hemostasis laboratories, where the more traditional and complex tests are still performed.
This article discusses currently available clinical tests of platelet function. Although some assays study global hemostasis as a whole, most platelet function assays target a specific phase of platelet function. Table 1 provides a detailed summary of currently available platelet function and hemostatic tests that depend to some extent on platelets, coupled with their clinical utility, advantages, and disadvantages.
| Name of Test | Principle | Advantages | Disadvantages | Frequency of Use | Clinical Applications |
|---|---|---|---|---|---|
| Adenine nucleotides | Measurement of total and released nucleotides by HPLC or luminescence | Sensitive | Sample preparation, assay calibration, extra equipment | Widely used in specialized laboratories, usually in conjunction with LTA | Diagnosis of storage and release defects |
| AspirinWorks | Immunoassay of urinary 11-dehydrothromboxane B 2 | Measures stable thromboxane metabolite, dependent on COX-1 activity | Indirect assay, not platelet specific, renal function dependent | Increasing use | Monitoring aspirin therapy and identifying poor responders at increased risk of thrombosis |
| Bleeding time | In vivo cessation of blood flow | In vivo test, physiologic POC | Insensitive, invasive, scarring, high CV | Was widely used, now less popular | Screening test |
| Blood smear | Microscopic analysis of blood cell on glass slide | Diagnostic | Artifacts can occur | Widely used | Detection of abnormalities in platelet size, number and granules, and leukocyte inclusion bodies |
| Clot retraction | Measures platelet interaction with fibrin | Simple | Nonspecific | Not widely used | Detection of abnormalities in integrin αIIbβ3 and fibrinogen |
| Combined aggregometry and luminescence | Combined WBA or LTA and nucleotide release | Monitors release reaction with secondary aggregation | Semiquantitative | Widely used in specialized laboratories, although less than LTA | Diagnosis of a wide variety of acquired and inherited platelet defects, diagnosis of storage and release defects |
| Electron microscopy | Ultrastructural analysis of platelets | Diagnostic Whole mount technique useful for dense granular imaging | Expensive, specialized equipment | Only available in special units | Detection of granular and ultrastructural defects, whole mount method can detect dense granular defects |
| Flow cytometry | Measurement of platelet glycoproteins and activation markers by fluorescence | Whole blood test, small blood volumes, wide variety of tests | Specialized operator, expensive, samples prone to artifact unless carefully prepared | Widely used | Diagnosis of platelet glycoprotein defects, quantification of glycoproteins, detection of platelet activation in vivo or in response to agonists, monitoring antiplatelet therapy (eg, VASP phosphorylation to monitor P2Y 12 inhibition) |
| Full blood count | Automated impedance and/or flow cytometry–based analysis of cells | Rapid, precise, provides platelet distribution and MPV | Less accuracy and precision at <20 × 10 9 /L | Widely used | Abnormalities in platelet number, size, and distribution; immature platelet fraction now available |
| Ichor Plateletworks | Platelet counting preactivation and postactivation | Rapid, simple, POC, small blood volume | Indirect test measuring count after aggregation | Used in surgery and cardiology | Monitoring antiplatelet drugs, prediction of bleeding |
| Impact cone and plate(let) analyzer | Quantification of high shear platelet adhesion/aggregation onto surface | Small blood volume required, high shear, rapid, simple, research (variable) and fixed versions available POC | Dependent on fibrinogen and VWF for adhesion of platelets | Mainly research but increasing use | Detection of inherited and acquired defects in primary hemostasis, detection of platelet hyperfunction, monitoring antiplatelet therapy |
| Kinetic aggregometer | Monitoring fluorescent platelet accumulation within a thrombus forming in a collagen-coated capillary | Sensitive to amplification pathways | Specialized laboratories only | Mainly Research | Monitoring antiplatelet therapy, detection of defects in primary hemostasis |
| Laser platelet aggregometer (PA-200) | Monitoring aggregation using a laser | Detection of microaggregates, sensitive | Little widespread experience | Little widespread experience | Detection of platelet hyperfunction |
| LTA | Low shear platelet-to-platelet aggregation in response to classic agonists | Gold standard | Time-consuming, sample preparation, poorly standardized | Widely used in specialized laboratories | Diagnosis of a wide variety of acquired and inherited platelet defects |
| 96 Well plate LTA (Optimul assay) | Similar to LTA but in 96 well plate | Lower blood/PRP volumes than LTA. Many replicates and dose response curves possible | Little widespread experience | Little widespread experience | Detection of defects in primary hemostasis |
| Microfluidic devices | Miniaturized multichannel devices | Whole blood, real-time thrombus formation | Little widespread experience | Research only at present | Detection of defects in primary hemostasis |
| PFA-100/200 | High shear platelet adhesion and aggregation during formation of a platelet plug | Whole blood test, high shear, small blood volumes, simple, rapid, POC | Inflexible, VWF-dependent, Hct-dependent, insensitive to clopidogrel | Widely used | Detection of inherited and acquired defects in primary hemostasis, monitoring aspirin, monitoring DDAVP therapy |
| PlaCor PRT | High shear thrombus formation in duplicate channels Blood oscillates through a narrowed channel with a spring to hold the thrombus | POC; finger prick blood | Little widespread experience | Little widespread experience | Rapid detection of SNPs and gene defects |
| Platelet genome or transcriptome analysis | Total mRNA content by microarray technology | Detection of all mRNAs in platelets and MKs | Instability of mRNA, possible impurity of starting material | Research only at present | Detection of novel gene mutations or transcription defects |
| Proteomic analysis | Measures total individual protein content | Sensitivity increasing | Loss of some membrane glycoproteins, possible impurity of starting material | Research only at present | Detection of protein deficiencies |
| Serum thromboxane B 2 | Immunoassay | Dependent on COX-1 activity | Prone to artifact, not platelet specific | Widespread use | Monitoring aspirin therapy, detection of thromboxane production defects |
| Soluble platelet release markers and sheddome (eg, PF4, βTG, sCD40L, sCD62P, GPV and GPVI) | Usually by ELISA | Relatively simple | Prone to artifact during blood collection and processing | Fairly widely used in research | Detection of in vivo platelet activation |
| Sonoclot analyzer | Impedance detection of changes of viscoelastic properties of celite-activated blood sample | Global test, POC, platelet count and function dependent | Measures clot properties only | Widely used in surgery and anesthesiology | Prediction of surgical bleeding, aid to blood product usage |
| Thromboelastography (TEG or ROTEM) | Monitoring rate and quality of clot formation | Global whole blood test, POC | Measures clot properties only, largely platelet independent unless platelet activators are used | Widely used in surgery and anesthesiology | Prediction of surgical bleeding, aid to blood product usage, monitoring rFVIIa therapy, new platelet mapping system can be used to monitor antiplatelet therapy (eg, aspirin or clopidogrel) |
| ThromboLUX | Dynamic light scattering of platelet quality | Simple, measures size and light intensity of platelets and microvesicles | Designed for platelet concentrates only | Little Widespread experience | Assessment of platelet concentrates |
| VASP | Flow cytometric measurement of vasodilator stimulated phosphoprotein | Measurement of P2Y 12 occupancy | Requires flow cytometer Insensitive to intermediate inhibition of P2Y 12 | Increasing use | Monitoring P2Y 12 receptor activity |
| VerifyNow | Fully automated platelet aggregometer to measure antiplatelet therapy | Simple, POC, 3 test cartridges (aspirin, P2Y 12 , and GP IIb-IIIa) | Inflexible, cartridges can only be used for single purpose | Increasing use | Monitoring antiplatelet therapy |
| WBA | Monitors changes in impedance in response to classic agonists | Whole blood test, multichannel version available. | Older instruments require electrodes to be cleaned and recycled | Widely used in specialized laboratories, although less than LTA | Diagnosis of a wide variety of acquired and inherited platelet defects |
Quality control, blood sampling, and anticoagulation
Platelet function testing presents many challenges in ensuring that accurate and meaningful results are obtained. Firstly, unlike with coagulation tests, there are no widely available internal or external quality control materials available. Most assays are performed on fresh blood, so many laboratories either establish normal ranges using control blood obtained from healthy volunteers or assay samples known to be normal in parallel to ensure that each test/reagent is viable.
Many platelet function tests, including the historical gold standard, light transmission aggregometry (LTA), remain poorly standardized. Among the variables, the most commonly cited are the range of agonists and agonist concentrations used, the choice of anticoagulant, and the method used to process the samples. The quality, handling, temperature, and age of a blood sample can cause significant artifacts in platelet analysis. For this reason, most laboratories test platelet function within a narrow time window (<2 hours), although some studies have shown that storage of blood up to 6 hours after drawing had a minimal effect on responses to all agonists, with the exception of ADP. Although the use of citrate is convenient within a coagulation laboratory, because citrate tubes are also used for clotting tests, anticoagulation of a blood sample through calcium chelation can immediately present a problem for platelet function testing because normal platelet function is largely calcium dependent. This problem can be overcome by use of nonchelating anticoagulants (eg, direct thrombin inhibitors, such as hirudin or lepirudin, or d -phenylalanyl- l -prolyl- l -arginine chloromethyl ketone [PPACK]). Because most platelet tests have been traditionally performed with citrated blood samples, however, it is often difficult to compare results between different studies using other anticoagulants. Handling of blood samples can also introduce artifactual activation or desensitization. Tests performed in whole blood present with immediate advantages but also with subtle differences compared with those that use platelet-rich plasma (PRP), which has to be prepared by centrifugation. Artifactual variables should be minimized during phlebotomy, anticoagulation, sample transit, and handling within a laboratory. There are several published guidelines that can help minimize platelet activation and platelet aggregation. These include using a light tourniquet, using a needle of at least 21 gauge, a nontraumatic venipuncture with smooth blood flow, discarding the first few milliliters of blood drawn, using polypropylene or siliconized glass tubes/syringes, ensuring immediate gentle mixing with anticoagulant, minimizing delays from sampling to analysis, keeping all tubes at room temperature, checking that blood tubes are not overfilled or underfilled, and avoiding unnecessary manipulation of the sample. These measures are most often used when performing the sensitive technique of measuring platelet activation by flow cytometry, but it could be argued that they should also be implemented when performing any platelet function testing. Many potential problems with samples are commonly overlooked and this may explain the wide interlaboratory variability reported for platelet function testing. Therefore, ensuring sample quality is of utmost importance before any platelet function testing is performed.
The in vivo bleeding time
Platelet function testing began with the application of in vivo bleeding time by Duke in 1910. The technique consists of inflicting a small incision in the skin of the forearm or the earlobe and recording the time required for a clot to form at the site of the incision and to stop the flow of blood. The bleeding time was further refined by the Ivy technique and the availability of commercial spring-loaded template disposable devices containing sterile blades (eg, Simplate II, Organon Teknika) ( Fig. 1 ). It was regarded as the most useful screening test of platelet function until the early 1990s.
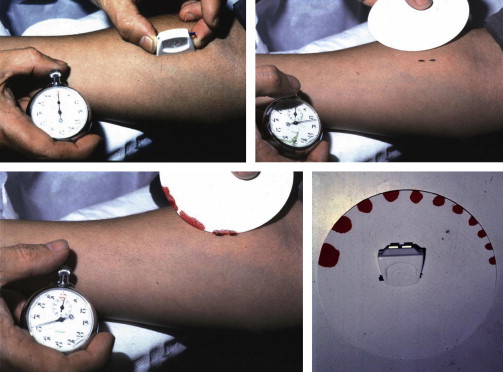
Advantages and Limitations of the Assay
The bleeding time test is simple; it measures physiologic hemostasis, including the role of the vessel wall components and does not require expensive equipment or a specialized laboratory. Despite its simplicity, the bleeding time is poorly reproducible, invasive, insensitive to many mild platelet defects, and time-consuming. In addition, the bleeding time fails to correlate with the bleeding tendency, and an accurate bleeding history is a more valuable screening test. As a consequence of these limitations, the widespread use of the bleeding time test has rapidly declined over the past 15 to 20 years, to be replaced by other less-invasive platelet function assays. Although a recent UK survey demonstrated that the bleeding time test is widely used in some centers, recent guidelines recommend that it should not be used as a screening test.
Light transmission aggregometry
LTA, also known as turbidimetric or optical aggregometry, was invented in the 1960s and soon revolutionized the identification and diagnosis of primary hemostatic defects. LTA is still regarded as the gold standard of platelet function testing and remains among the most used in specialized laboratories for the identification and diagnosis of many platelet defects. Briefly, platelet aggregation is measured by analysis of the transmission of light through a sample of PRP obtained by centrifuging anticoagulated whole blood at low g-force. By definition, PRP is a turbid suspension of cells, which significantly reduces light transmission. After addition of a platelet agonist, formation of aggregates reduces the turbidity of the suspension, resulting in increased light transmission (100% light transmission is set with platelet-poor plasma [PPP]). The dynamics of platelet aggregation (expressed as a percentage) are, therefore, measured in real time as platelets aggregate. In recent years, commercial aggregometers have become easier to use with multichannel capability, simple automatic setting of 100% and 0% baselines, and computer operation and storage of results ( Fig. 2 ). Some lumiaggregometers can also measure the secretion of nucleotides during aggregation by simultaneously measuring luminescence.

Advantages and Limitations of the Assay
Platelet activation and ensuing aggregation measured by LTA can be induced by many agonists and agonist concentrations. As a result, LTA offers the possibility of studying platelet activation pathways in detail and has been the preferred platelet function assay in most specialized clinical laboratories over the past 50 years because it correlates with bleeding and ischemic outcomes.
Although considered the most useful diagnostic and research tool, LTA is nonphysiologic, because separated platelets are usually stirred under low shear conditions during the test and only form aggregates after addition of agonists, conditions that do not accurately mimic platelet adhesion, activation, and aggregation on vessel wall damage. As with most platelet function assays, results are influenced by platelet count, making it unsuitable in severely thrombocytopenic patients. Also, conventional LTA using a full panel of agonists requires large blood volumes and significant expertise to perform the tests and to interpret the tracings, triggering the development of new, easier-to-use platelet function assays (see Table 1 ).
Clinical Applications
Most laboratories perform LTA with a panel of different concentrations of classical agonists (eg, ADP, collagen, epinephrine, arachidonic acid, and ristocetin) and sometimes an extended panel of agonists (thrombin receptor activating peptide [TRAP], collagen-related peptide, calcium ionophore, thromboxane mimetics, such as U46619, and so forth), although the exact range of agonists and their concentrations remain poorly standardized between laboratories. Nonetheless, LTA is still regarded as the gold standard for platelet function testing and remains the most useful technique for diagnosing a wide variety of platelet defects as well as monitoring antiplatelet therapy. Recent efforts to standardize LTA have been published and are available online from the International Society on Thrombosis and Haemostasis Platelet Physiology Subcommittee of the Scientific and Standardization Committee.
Modified 96 well plate assay for platelet aggregation
In an attempt to reduce the blood volume necessary for assessment of platelet aggregation yet retain the flexibility of LTA derived from testing multiple platelet activation pathways with specific agonists, several laboratories have developed a modified technique based on light transmission principles but applied to standard 96 well plates. The technique requires the same preparatory steps as LTA (ie, isolation of PRP and PPP from whole blood by centrifugation) and, therefore, has similar applications and restrictions. Addition of PRP to wells with platelet agonists either precoated onto the plate or in solution triggers platelet activation and aggregation when the plate is stirred, which changes the absorbance of light passing through the 96 well plate assay. The assessment of platelet function is, therefore, simply assessed by measuring absorbance in a conventional plate reader, which most laboratories possess. Absorbance can be measured either kinetically or as an endpoint (eg, after 5 minutes) and is then converted into percentage of aggregation in similar fashion to LTA based on measuring both control PRP and PPP samples.
Advantages and Limitations of the Assay
Just as for LTA, platelet activation and ensuing aggregation can be induced by numerous agonists at varying concentrations, which allows for extensive testing of the different platelet activation pathways. The major advantage of this assay, however, is a drastic reduction in the volume of blood/PRP required and the time required to run the test. Because all agonists are studied simultaneously in up to 96 wells, this technique can provide a rapid assessment of most platelet activation pathways within minutes, including detailed dose-response curves (with replicates), which are rarely obtainable from LTA due to blood volume restrictions, the number of channels available, and time. The availability of standardized lyophilized 96 well plates with a range of agonists at standard concentrations available from a central source could also improve the interlaboratory variation of this assay. Results also can potentially be uploaded to a central server to check quality control of each assay and calculate the results online.
The assay remains experimental and clinical experience with this assay is limited. As a consequence, how the 96 well plate assay compares with classical LTA is not known. Because of the similarities between the 2 assays, the limitations of LTA also apply to the 96 well plate assay, except the time requirement for an experienced operator.
Clinical Applications
In theory, clinical applications for the 96 well plate assay would be similar to LTA; however, this has not yet been tested in large clinical studies. In a research setting, the assay seems promising for the clinical diagnosis of bleeding disorders as well as for monitoring antiplatelet therapy.
Whole blood aggregometry
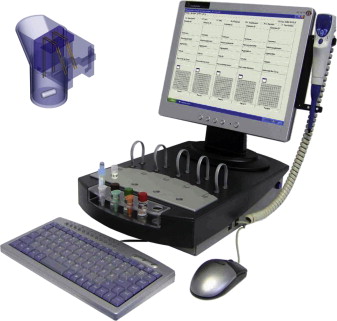
Whole blood aggregometry (WBA) provides a means to study platelet function within anticoagulated whole blood without any sample processing. The test measures the change in resistance or impedance between 2 electrodes as platelets adhere and aggregate in response to classical agonists. The original Chrono-Log instrument was a 2-channel device with luminescence capability, which has been updated to become a fully computerized 2-channel or 4-channel instrument ( Fig. 3 ). Although the earlier versions of this apparatus required electrodes to be carefully cleaned to remove platelet aggregates after each use, the technology now allows for disposable single-use electrodes.

In recent years, a new 5-channel, computerized WBA instrument (Multiplate, Verum Diagnostica) ( Fig. 4 ) has gained general favor in clinical laboratories, because it is fully automated and uses disposable cuvettes/electrodes with a range of diverse agonists for different applications, including diagnosis of bleeding and monitoring antiplatelet therapy. Although the basic methodologies are similar, the way results are reported are different between the Chrono-Log (in Ω, the maximal amplitude of impedance achieved) and Multiplate (in arbitrary units, as area under the curve achieved over 6 minutes of aggregation).