Targeted Therapies for the Treatment of Advanced Colorectal Carcinoma
Allyson J. Ocean
Romae Palmer
Manish A. Shah
Colorectal cancer is the second most common malignancy in the United States and the third most common cancer worldwide. Approximately 143,000 individuals were diagnosed with colorectal cancer in the United States in 2012 and 51,000 died of the disease.1 Nearly 80% of tumors are limited to the colorectum and/or regional lymph nodes at the time of diagnosis and, thus, are potentially amenable to surgical resection with a chance for cure. The 5-year relative survival rates are 58% to 97% following surgery for disease limited to the colorectum without regional lymph node metastases but drop to 30% to 70% for stage III disease, in which case lower survival rates inversely correlate with the extent of regional lymphadenopathy. Twenty percent of patients present with distant metastatic disease (stage IV) which is associated with a 10% 5-year survival rate. Long-term survival is mostly limited to patients with disease amenable to resection of metastatic deposits.2,3 Treatment advances include the development of novel biologic therapeutic agents and their increased application to advanced tumors, as well as an increased proclivity toward surgical resection of tumor deposits in distant metastatic sites. These approaches may be used in combination to maximize survival and minimize morbidity of colorectal cancer patients. Emerging techniques have identified molecular markers of colorectal cancer that represent potential drug targets, thereby allowing physicians to tailor specific therapies and personalize patient treatment. The purpose of this chapter is to provide information regarding important treatment aspects in colorectal cancer with a focus on the most recent advances in chemotherapy, particularly targeted therapies.
CYTOTOXIC THERAPIES FOR MANAGEMENT OF COLORECTAL CANCER
The Food and Drug Administration has approved nine drugs for the treatment of colorectal cancer. Four of these are cytotoxic chemotherapy agents: 5-fluorouracil (5-FU), oxaliplatin, irinotecan, and capecitabine. Bevacizumab, cetuximab, panitumumab, and ziv-aflibercept are approved for treatment of advanced colorectal cancer when used in combination with conventional chemotherapy, whereas cetuximab, panitumumab, and regorafenib are approved as single agents in the treatment of this disease.
5-Fluorouracil
The pyrimidine analog 5-FU is an antimetabolite that works via noncompetitive inhibition of thymidylate synthase. Folinic acid (leucovorin) is usually given in combination with 5-FU to enhance its cytotoxicity by increasing the formation of ternary complexes with thymidylate synthase.4 The specific metabolites of fluorouridine triphosphate and fluorodeoxyuridine monophosphate
are incorporated into RNA and DNA, respectively, through ribosylation and phosphorylation. Five-fluorouracil is an S phase-specific drug that induces cell cycle arrest and apoptosis that is administered as a continuous infusion.5 It is usually administered in combination with other cytotoxic chemotherapy, such as oxaliplatin (i.e., 5-FU, leucovorin, and oxaliplatin [FOLFOX] regimen) or irinotecan (i.e., 5-FU, leucovorin, and irinotecan [FOLFIRI] regimen), although it may also be administered as a single intravenous agent or directly infused into the liver or peritoneal cavity.
are incorporated into RNA and DNA, respectively, through ribosylation and phosphorylation. Five-fluorouracil is an S phase-specific drug that induces cell cycle arrest and apoptosis that is administered as a continuous infusion.5 It is usually administered in combination with other cytotoxic chemotherapy, such as oxaliplatin (i.e., 5-FU, leucovorin, and oxaliplatin [FOLFOX] regimen) or irinotecan (i.e., 5-FU, leucovorin, and irinotecan [FOLFIRI] regimen), although it may also be administered as a single intravenous agent or directly infused into the liver or peritoneal cavity.
Oxaliplatin
Oxaliplatin is a third-generation platinum analog that covalently binds DNA and kills tumor cells in all stages of the cell cycle. This agent binds DNA through intra- and interstrand cross-links that inhibit DNA synthesis and functionality. Platinum analogs also bind to both cytoplasmic and nuclear proteins, thereby enhancing their cytotoxic effects. The major route of platinum elimination is renal excretion, and thus, clearance correlates with glomerular filtration rate. Peripheral neuropathy is a major toxicity of oxaliplatin and manifests as an acute and/or chronic injury. Acute neurologic toxicity is reversible and often triggered by exposure to cold temperatures. It presents as transient paresthesia, dysesthesia, or hypoesthesia in the hands, feet, perioral skin, or laryngopharyngeal space. Chronic neurologic toxicity develops after cumulative doses and manifests as persistent, sometimes irreversible, neuropathy and/or deficits in proprioception that interfere with activities of daily living.
Oxaliplatin was initially used as a component of second-line therapy.6 However, emerging data suggest that the combination of oxaliplatin and 5-FU is superior to 5-FU and leucovorin with respect to tumor response rate, progression-free survival, and overall survival as a first-line therapy for advanced colorectal cancers and metastatic disease.7,8 Oxaliplatin is inactive as a single agent and should be administered only in combination with other chemotherapeutic agents, such as 5-FU or irinotecan. Either combination may be administered with bevacizumab or epidermal growth factor inhibitors.
Irinotecan
Irinotecan is a derivative of camptothecin that is isolated from the Camptotheca acuminate tree. It is a topoisomerase I inhibitor and S phase-specific agent that binds to the topoisomerase I-DNA complex. Irinotecan is the water-soluble precursor of the lipophilic metabolite SN-38, which is approximately 1,000 times more potent than irinotecan as an inhibitor of topoisomerase I. Irinotecan is active as monotherapy for patients with advanced colorectal carcinoma, although it is more effective in combination with 5-FU and targeted agents, such as bevacizumab, cetuximab, and panitumumab.
Addition of irinotecan to 5-FU and leucovorin improves tumor response and survival compared to 5-FU and leucovorin alone.9, 10 and 11 Different administration schedules (weekly, every 2 or 3 weeks) result in similar therapeutic outcomes. The most severe dose-limiting toxicity is diarrhea, which may be debilitating, although myelosuppression may also occur. Early use of loperamide decreases the severity of diarrhea and is essential to prevent treatment-related morbidity and mortality. Administration on a 3-week schedule is associated with significantly less severe diarrhea than more frequent doses.12
Capecitabine
Capecitabine is an orally administered fluoropyrimidine carbamate that is absorbed through the gastrointestinal tract and converted to 5-FU via three sequential enzymatic reactions, the last of which involves thymidine phosphorylase. This enzyme is present at consistently higher levels in tumor cells compared with nonmalignant tissue, thereby enhancing selectivity of this agent for tumor cells and improving its tolerability among patients compared to 5-FU.13 Capecitabine monotherapy is similarly effective as a first-line treatment of metastatic colorectal cancer compared to 5-FU and leucovorin.14,15 Capecitabine can be combined with oxaliplatin (XELOX) to achieve similar efficacy and tolerability compared to other 5-FU/oxaliplatin combinations and may be used as a convenient alternative to the latter.16, 17 and 18
TARGETED AGENTS IN METASTATIC COLORECTAL CANCER
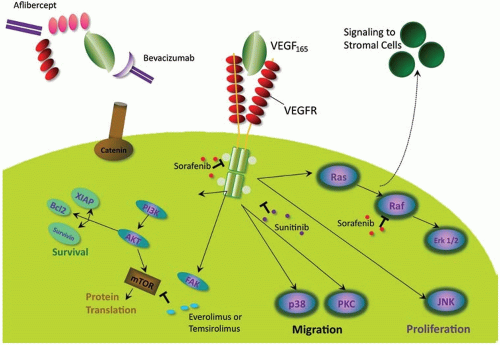
Vascular Endothelial Growth Factor
Angiogenesis is essential for the advancement of tumor growth, supplying nutrients and oxygen and removing of waste products, but it is also a key factor in promoting cell migration and development of distant metastases.19 Vascular endothelial growth factor (VEGF) is a diffusible glycoprotein expressed by both endothelial cells and tumor cells that plays a pivotal role in regulating angiogenesis.20,21 There are several different isoforms of the receptor: VEGF-A, VEGF-B, VEGF-C, VEGF-D, and VEGF-E. VEGF-A is the most potent stimulator of angiogenesis and binds to two related tyrosine kinase receptors: VEGFR-1 and VEGFR-2.20,21 Binding of a VEGF isoform to one, or both, of its receptors results in activation of a cell type-dependent signaling cascade leading to neovascularization, mitogenesis, and suppression of apoptosis (Figure 16.1). Inhibition of VEGF reduces the rates of tumor growth, metastases, tumor vascularity, and the permeability of tumor microvessels.22 Expression of VEGF correlates with development of colorectal cancer metastases and early recurrence.23
Bevacizumab
Antiangiogenic agents have a high binding affinity to VEGF and interfere with binding of VEGF-A to VEGFR-1 and VEGFR-2, thereby inhibiting VEGF-mediated intracellular signaling.24 Bevacizumab is a monoclonal antibody with high affinity for VEGF-A. It sequesters VEGF-A and prevents its binding to VEGFR, thereby preventing endothelial cell signaling.19 Bevacizumab has minimal efficacy as a single agent and is most commonly administered in combination with cytotoxic chemotherapy. Enhanced antitumor activity presumably results from bevacizumab-mediated inhibition of tumor neoangiogenesis, tumor growth, and the development of new metastasis. These effects reduce intratumoral hydrostatic pressure, thereby promoting delivery of cytotoxic chemotherapy to tumor cells.25
Data from several studies clearly demonstrate the efficacy of bevacizumab in the treatment of stage IV colorectal cancer, although it is not associated with substantial benefit when used to treat patients with stages II and III colorectal cancer (Table 16.1).21,26, 27




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree