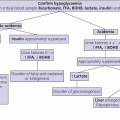
4 Tall stature is defined as height greater than two standard deviations above the mean (equivalent to >97th centile) for the general population. It may accompany disorders such as sexual precocity and simple obesity, and not be the prime concern. When tall stature per se is the principal reason for referral, the family will wish an estimate of final height and possible treatment to be discussed. Tall stature is a much less common cause of referral than short stature, being more acceptable to families than the latter unless the degree of tallness is particularly marked, especially in a girl. Tall stature is usually associated with one of four broad aetiological categories. In order of frequency these are: (i) familial tall stature/constitutional advance in growth; (ii) simple obesity; (iii) syndromic tall stature, which has usually been present since infancy; and (iv) endocrine causes. The differential diagnosis is shown in Table 4.1 and a helpful diagnostic algorithm is also recommended (Davies and Cheetham 2014). The European Society for Paediatric Endocrinology (ESPE) provides a more comprehensive classification (Wit et al. 2007). Table 4.1 Differential diagnosis of tall stature. Most tall stature conditions are rare. An idea of their relative frequency as the principal reason for referral can be gained by examining the following breakdown of referrals to one consultant in Glasgow from 1989 to 2009: normal genetic tall stature (81); constitutional advance in growth and adolescence (49); tall stature due to simple obesity (70); dysmorphic syndromes: 47,XYY (3), 47,XXY (11), 47,XXX (1), Marfan syndrome (18), Sotos syndrome (5), Weaver syndrome (1), Beckwith‐Wiedemann syndrome (3), unclassified dysmorphic syndromes (22); sexual precocity (19); thyrotoxicosis (2), and growth hormone excess (2). The key aspects of clinical assessment of a child or adolescent referred with tall stature are given in Table 4.2. With a combination of auxology, careful history taking, and physical examination followed by serial measurements of height, laboratory investigations will not usually be needed. If there are dysmorphic features, particularly in the context of learning difficulties, a clinical geneticist should be invited to see the child and advise on specific molecular genetic studies. Since the phenotype of Marfan syndrome (see Table 4.5) can be mild, it is wise to carry out a screening cardiac ultrasound in children who are taller than expected for parental heights, or in whom one parent is particularly tall. Table 4.2 Clinical assessment of tall stature. Children whose height falls above the parental target range centiles in the absence of a family history of constitutional advance and/or an enhanced growth rate will require investigation, as shown in Table 4.3. The combination of tall stature and developmental delay should always prompt a search for an underlying syndrome. If physical examination is completely normal and the child comes from a tall family, investigations are usually not indicated and regular height measurements will be sufficient. Further investigations will be dictated by the results of the baseline tests. If the rare diagnosis of excess growth hormone (GH) secretion caused by a GH‐secreting pituitary adenoma is considered, investigation should proceed as shown in Table 4.4. Table 4.3 Baseline investigations for tall stature. Table 4.4 Assessment for possible growth hormone excess. This test involves giving 1.75 g/kg carbohydrate orally (up to maximum of 75 g = two glasses of Lucozade) as for the glucose tolerance test, and measuring serum GH at: ‐30, 0, 30, 60, 90, 120 and 150 minutes together with an insulin‐like growth factor IGF‐I level at baseline. Normal GH suppression is a value of <4 mU/L at some time during the test. NB: It is wise to put in the request for this test as ‘GH suppression test with glucose’ rather than ‘glucose tolerance test’ – if the latter term is used, the day ward is likely to take samples for glucose and not GH! Physiological tall stature can be sub‐classified into familial tall stature and constitutional advance in growth – the counterpart of familial short stature and constitutional delay (see Chapter 3). As with familial short stature and constitutional delay, there is considerable overlap between familial tallness and constitutional advance. Also, as with short stature, the clinician needs to guard against the pitfall of assuming that tallness in one parent is necessarily normal – dominantly inherited tall stature disorders (e.g. Marfan syndrome) can be mistaken for normal tall stature. Normal tall stature is the most common cause of referral in tall children and adolescents, and the patient is usually a girl. In this case, it is frequently the mother who, having suffered herself from being unusually tall as a child and adolescent, is concerned about her daughter. In groups of tall children, GH secretion has been shown to be statistically elevated when compared to children with normal or short stature. However, in an individual child, excess GH secretion is not usually apparent. The tall stature is usually noticeable by primary school entry. It is helpful to ask the parents about their childhood height and age of onset of puberty. The history of one parent being particularly tall as a child, having an early adolescence, and being relatively less tall as an adult, is indicative of familial constitutional advance, and the diagnosis is supported by bone age advance in the child. Physical examination is normal although advanced adolescence may be noted in girls >9 years. In girls and boys with normal tall stature, prediction of final height from skeletal maturity and current height may be helpful from around 11 years upwards. However, the accuracy of final height prediction in tall stature varies between populations and with the prediction method used, hence should not be used to assess any treatment effect. The likelihood of an earlier‐than‐average puberty should be explained to parents whose children are still prepubertal. Reassurance and growth monitoring are the principles of management. Children with simple (exogenous) obesity are relatively tall in relation to their parental height centiles, with increased height velocity and moderately advanced bone age (Buckler 1994). This increase in height status may, depending on familial heights, be sufficient to put the child above the 97th centile for height, or to compound existing tall stature. The combination of tall stature and developmental delay should always prompt a search for an underlying dysmorphic syndrome. Boys with learning difficulties should be screened for Fragile X syndrome irrespective of stature. The typical phenotype in this condition includes long face, large and prominent ears, increased head size, and (especially after puberty) enlarged testes. Of the aneuploidy syndromes, the 47,XYY syndrome is the most likely to present as tall stature (with long legs) and, in most cases, both phenotype and intelligence are normal although some individuals show behavioural problems. The principal phenotypic features of three important tall stature syndromes which may present to the growth clinic – Marfan, Sotos, and Beckwith‐Wiedemann syndrome – are given in Table 4.5. Table 4.5 Principal features of Marfan, Sotos, and Beckwith‐Wiedemann syndromes. Marfan syndrome and related disorders are a complex and phenotypically variable group of conditions caused by mutations in the FBN1 gene which encodes fibrillin‐1 (Pepe et al. 2016). In suspected cases, the revised Ghent scoring system (Loeys et al. 2010) is useful in helping both to avoid over‐diagnosis and to distinguish classic Marfan syndrome from allied conditions, such as the mitral valve prolapse, aortic enlargement, skin and skeletal (MASS) variant. In MASS, lens dislocation does not occur and the risk of aortic dissection is lower. Sotos syndrome is considered when tall stature is accompanied by mild learning difficulties and characteristic facial/skull appearance. Bone age is usually advanced so that adult final height is not markedly increased, with values of 184.3 cm for men and 172.9 cm for women in one study (Agwu et al. 1999). The typical genetic defect is in the NSD1 gene, but Sotos has reported a new form of the condition caused by heterozygous mutations in the Nuclear Factor 1,X type ( NFIX ) gene on 19p13.3 (Sotos 2014). In Beckwith‐Wiedemann syndrome, tall stature is an accompanying rather than presenting feature. Diagnosis is important so that appropriate management and surveillance can be implemented, particularly for tumour development (Weksberg et al. 2010) Sexual precocity, thyrotoxicosis, and familial glucocorticoid deficiency (see Chapter 8) do not usually present with tall stature alone, while aromatase deficiency/oestrogen receptor defects are extremely rare. Children with suspected GH excess will require hormone investigations to confirm the abnormality; specialist referral at this stage is recommended. A GH‐secreting tumour causes tall stature and gigantism in childhood and adolescence, and acromegaly in adult life. An association with McCune–Albright syndrome is recognized. Because of its extreme rarity in childhood, this disorder should be managed jointly with an adult endocrinologist with experience of managing acromegaly. Management of pituitary gigantism should be according to the Endocrine Society guidelines (Katznelson et al. 2014). Trans‐sphenoidal surgery is the treatment of choice, with either medical therapy or radiotherapy (preferably stereotactic) if tumour removal is incomplete or not feasible. Medical therapy comprises daily subcutaneous injections of the growth hormone receptor antagonist Pegvisomant, which has been shown to decrease height velocity in gigantism. This may be used in conjunction with the long‐acting preparation of the somatostatin antagonist Octreotide which is initially given as a deep subcutaneous injection every month. This is a controversial area. In the US, very few otherwise healthy girls are currently treated with high dose oestrogen to reduce adult height. This is partly because tall stature is now seldom regarded as a problem requiring intervention. Moreover, apart from ethical concerns about intervention in a physiological situation, there are also long‐term health concerns (see below). Despite this, the degree of anxiety surrounding advanced growth, may be sufficient for treatment to be given. The aim is to try to slow down growth and therefore reduce final height by reducing the prepubertal rather than the pubertal component of growth, Three forms of therapy have either been used or are under consideration: The indication for the use of sex steroids to reduce final height is based on evidence that abnormally high circulating levels will advance skeletal maturation and eventually cause early epiphyseal fusion. The most extensive results have been published by a group from the Netherlands (De Waal et al. 1996). In girls, Ethinylestradiol 100–300 μg/day orally combined with cyclical progesterone (i.e. norethisterone 5 mg/day for days 1–14 of each calendar month), reduced final height by up to 7 cm. In boys, testosterone 250–1000 mg monthly caused a similar reduction. An early age of onset of treatment (bone age 10 years in girls, 12.5 years in boys) was associated with the best results. However, further work from the Netherlands has raised fertility concerns following high dose oestrogen treatment in childhood and adolescence. A study on 125 tall women found time to first pregnancy to be increased in the 95 subjects who had been treated with high dose Ethinylestradiol compared with the untreated women. Also, those treated with 200 μg daily took longer to conceive than the women receiving 100 μg, suggesting a dose effect (Hendriks et al. 2012). In Glasgow, our experience of tall stature treatment has been virtually limited to selected girls with Marfan syndrome, using lower doses (50–100 μg daily) of Ethinylestradiol (Kalkan Ucar et al. 2009). In boys, high dose intramuscular testosterone therapy (250 mg every two weeks) can be given to accelerate epiphsyeal fusion but, in practice, few centres employ this strategy. Treatment with Lanreotide, a long‐acting preparation of the somatostatin analogue Octreotide, has been reported (Carel et al. 2009). The GH receptor antagonist Pegvisomant is known to reduce growth velocity in pituitary gigantism and would thus be expected to reduce final height in tall girls and boys. However, experience remains limited and not all clinicians are comfortable giving therapy of this invasive nature to essentially normal subjects. At present we advise against use of either treatment unless the circumstances are exceptional. Where possible, such experimental therapy should be conducted within a prospective research setting. Epiphysiodesis, in which the knee epiphyses are ablated by percutaneous drill and curettage, is an established treatment for leg length inequality, the longer limb being treated. This technique has also been applied to tall girls and boys. A study from Sweden reported the outcome of bilateral percutaneous epiphysiodesis in 12 girls and 9 boys with tall stature in which final height was 4.1 and 6.4 cm below the predicted height (Benyi et al. 2010). The long‐term safety of this technique remains unclear. Also, assessment of its efficacy is difficult because of small patient numbers, and the questionable validity of the height prediction model in exceptionally tall (and short) subjects.
Tall Stature
Pathogenesis and differential diagnosis
Assessment of the child or adolescent with tall stature
Auxology
Height, weight, height velocity
Heights of parents and siblings
Bone age and (if appropriate) height prediction
History
Pattern of growth including age when tall stature recognized
Birth weight, head circumference and (if available) birth length
Family patterns of childhood growth and puberty
Neurodevelopment, academic performance and behaviour
Examination
Presence of dysmorphic features
Pubertal staging including assessment of testicular volume
Fundoscopy to assess optic discs
Systematic examination
Investigations
Cardiac ultrasound
Karyotype
Fibrillin‐1 gene for Marfan syndrome
FMR1 gene analysis for Fragile X syndrome in boys with tall stature and learning difficulties
DNA for specific syndromes (after assessment by clinical geneticist)
Thyroid function tests
IGF‐I
Bone age assessment and (where appropriate) height prediction
GH suppression test using oral glucose load
MRI of hypothalamus and pituitary
Visual fields
9.00 a.m. cortisol
Prolactin
Testosterone, LH, FSH (depending on age)
Protocol for GH suppression test with glucose
Causes of tall stature
Familial tall stature and constitutional advance in growth and adolescence
Simple obesity
Syndromes associated with tall stature
Marfan syndrome
Genetics
Clinical features
Diagnosis
Clinical, supported by cardiac ultrasound and eye findings, confirmed by genetic studies
N.B.: Phenotype may be mild.
Sotos syndrome
Genetics
Clinical features
Diagnosis
Beckwith–Wiedemann syndrome
Genetics
Clinical
Diagnosis
Endocrine causes of tall stature
Pituitary gigantism
Treatment of familial (constitutional) tall stature
Sex‐steroid therapy
Somatostatin analogue and GH receptor antagonist therapy
Surgical treatment
Future developments
When to involve a specialist centre
Controversial points
Potential pitfalls
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree