Pulmonary embolism
Cerebral vein thrombosis
Thrombosis and thromboembolism
Number
Rate
95 % CI
Number
Rate
95 % CI
Number
Rate
95 % CI
1985–1987
30
1.32
0.83
1.89
2
0.09
0.02
0.32
32
1.41
1.00
1.99
1988–1990
24
1.02
0.68
1.51
9
0.38
0.20
0.72
33
1.40
1.00
1.96
1991–1993
30
1.30
0.91
1.85
5
0.22
0.09
0.51
35
1.51
1.09
2.10
1994–1996
46
2.09
1.57
2.79
2
0.09
0.02
0.33
48
2.18
1.65
2.90
1997–1999
31
1.46
1.03
2.07
4
0.19
0.07
0.48
35
1.65
1.19
2.29
2000–2002
25
1.25
0.85
1.85
5
0.25
0.11
0.59
30
1.50
1.05
2.14
2003–2005
33
1.56
1.11
2.19
8
0.38
0.19
0.75
41
1.94
1.43
2.63
2006–2008
16
0.70
0.43
1.14
2
0.09
0.02
0.35
18
0.79
0.49
1.25
The incidence of deep vein thrombosis (DVT) in pregnancy and the puerperium is approximately 1 in 1000 pregnancies [20]. VTE can occur at any stage of pregnancy but the puerperium is the time of highest risk, with estimates of relative risk of approximately 20-fold [21]. In pregnancy, DVT occurs more commonly in the left leg (up to 90 %) in contrast to 55 % in the non-pregnant state. This observation may be explained by compression of the left common iliac vein by the gravid uterus, at the point where it is crossed by the overlying right common iliac artery (May-Thurner syndrome). However, this hypothesis would not seem to apply not seem to apply to DVT observed in early pregnancy. A systematic review of 6 studies, which included 124 patients, selected because they provided objective diagnostic and anatomic information for unselected or consecutive symptomatic pregnant patients with DVT, identified that proximal DVT restricted to the femoral or iliac veins occurred in over 60 % of cases [22].
The risk factors for VTE in pregnancy are shown in Table 5.2. The prevalence of many of these risk factors is increasing. For example, levels of obesity in the general and pregnant populations are rising in the UK and elsewhere. The average age of pregnancy is rising constantly and, related to this, there are more pregnant women with coexisting medical morbidities such as heart, lung, or bowel disease. Because of the increasing availability of assisted reproduction technologies, multiple pregnancy is also on the rise. It is to be hoped that more widespread recognition and assessment of these risk factors will lead to more consistent use of thromboprophylactic measures (see Chap. 4).
Table 5.2
Risk factors for venous thromboembolism in pregnancy and the puerperium
Pre-existing | New onset or transient |
|---|---|
Age over 35 years | Surgical procedure in pregnancy or puerperium, e.g. evacuation of retained products of conception, postpartum sterilization, emergency CS, elective CS with other risk factors |
Obesity (Weight >100 kg or BMI >30 kg/m2) either pre-pregnancy or in early pregnancy | Hyperemesis |
Parity ≥3 | Dehydration |
Smoker | Ovarian hyperstimulation syndrome |
Gross varicose veins with phlebitis | Multiple pregnancy or assisted conception |
Paraplegia | Severe infection, e.g. pyelonephritis |
One or more significant medical co- morbidities, e.g. heart disease; metabolic, endocrine or respiratory pathologies; inflammatory conditions (e.g. inflammatory bowel disease) | Immobility (e.g. SPD, significantly reduced mobility for 3 or more days; long distance travel >4 h during pregnancy and up to 6 weeks post- partum) |
Known thrombophilias and other thrombotic conditions, e.g. hemoglobinopathies, myeloproliferative disease (essential thrombocythemia, polycythemia vera), nephrotic syndrome | Preeclampsia |
Excessive blood loss | |
Prolonged labor (>24 h) | |
Mid-cavity instrumental delivery | |
Immobility after delivery | |
Critical care admission | |
PPH >1 L or blood transfusion |
Most women who die from PE will have identifiable risk factors. In the CMACE report of 2006–2008 [1], 16 of the 18 women who died from PE in the UK had recognized risk factors; and in the UKOSS report of 143 antenatal PE, including 5 fatal events, 70 % had identifiable risk factors [16].
Compared with VTE, arterial thrombosis is far less common in pregnancy, possibly reflecting the lower incidence of atherosclerotic plaques in women of this age. The incidence of stroke in pregnancy is reported as 0.04–0.34 per 1,000 births [23–26] and myocardial infarction 0.1 per 1,000 births [27]. However, a proportion of stroke will be hemorrhagic, and myocardial infarction may be due to coronary artery dissection (the risk of which is increased in pregnancy). Some of the risk factors for VTE also increase the risk of arterial thrombosis; these include older age, obesity and smoking. The risk of arterial thrombosis is also increased in association with hemoglobinopathies, antiphospholipid syndrome and other acquired disorders including myeloproliferative neoplasms and paroxysmal nocturnal hemoglobinuria, and with drugs that can cause arterial spasm such as ergometrine, cocaine and marijuana.
5.3 Pathophysiology of Venous Thromboembolism
The pathophysiology of VTE has classically been described in terms of Virchow’s triad of venous stasis, hypercoagulability, and vascular damage. All three of these components are affected during pregnancy. Because of the vasodilator effect of progesterone, relaxin, and other pregnancy-related hormones, and because of the physical obstruction of the gravid uterus, there is increased venous stasis in the pelvic and lower limb veins. Doppler studies of venous blood flow in the lower limbs in pregnancy show that venous flow velocity is reduced by 50 % by the end of the second trimester and reaches a nadir at 36 weeks’ gestation [28]. After delivery, flow velocity takes 6 weeks to return to normal. Pregnancy is a hypercoagulable state (as detailed in Chap. 1); hemostatic changes in coagulation factors, the fibrinolytic system, and natural anticoagulants prepare the body for the challenges of implantation, placentation, and delivery. A number of coagulation factor levels increase, including fibrinogen and factor VIII as well as von Willebrand factor, whilst other coagulation factor levels remain unchanged or decrease. The rise in factor VIII leads to a shortened activated partial thromboplastin time (APTT) in late pregnancy. These increased coagulation factors are not balanced by increases in the naturally occurring anticoagulants. Both free and total protein S decrease during pregnancy. Although protein C levels remain normal or show a slight increase, there is an increase in activated protein C resistance (APCR), largely due to the increase in factor VIII and decrease in protein S levels. Antithrombin generally remains unchanged. Thrombin generation increases during pregnancy and, although global fibrinolytic activity is reduced, plasma D-dimer (a marker of activation of fibrinolysis secondary to coagulation activation) increases [29–33]. Finally, delivery, whether vaginal (normal or instrumental) or abdominal (Cesarean section), inevitably causes a degree of injury to pelvic vessels.
5.4 Diagnosis of Venous Thromboembolism
The clinical diagnosis of acute VTE is often difficult, particularly in pregnancy when edema of the lower limbs is common, as is dyspnea (which occurs in up to 70 % of all pregnant women). The accuracy of clinical diagnosis of VTE is very low (approximately 8 % for DVT and 5 % for PE) [21, 34, 35]. It is important, therefore, to maintain a high index of suspicion in women presenting with some or all of the typical symptoms or signs of DVT (leg pain and swelling, usually unilateral lower abdominal pain) or PE (dyspnea, chest pain, hemoptysis, low grade pyrexia, collapse) or CVT (headache, clouding of consciousness or confusion, or other neurological symptoms). Any woman with suggestive symptoms and signs should undergo objective testing to confirm or rule out the diagnosis. Until the diagnosis is excluded, the woman should be started on a treatment dose of low-molecular-weight heparin (LMWH), unless there is a strong contraindication to anticoagulation. The symptoms and signs of VTE are summarized in Table 5.2, and differential diagnoses in Table 5.3.
Table 5.3
Symptoms and signs of venous thromboembolism in pregnancy
Deep vein thrombosis |
Painful warm leg |
Swelling |
Erythema |
Tenderness |
Lower abdominal pain |
Pulmonary embolism |
Pleuritic chest pain |
Dyspnea |
Tachypnea |
Cough |
Hemoptysis |
Tachycardia |
Raised jugular venous pressure |
Focal chest signs |
Collapse |
Shock |
Cerebral vein thrombosis |
Headache |
Vomiting |
Photophobia |
Seizures |
Impaired consciousness |
Focal neurological signs |
5.4.1 Diagnosis of Deep Venous Thrombosis
When there is a clinical suspicion of DVT, the woman should undergo a venous duplex or compression ultrasound scan of the whole lower limb as soon as possible. If the scan confirms the presence of a DVT, anticoagulation therapy should be continued. If the scan is negative and clinical suspicion is low, anticoagulation should be stopped. However, if the scan is negative but the clinical suspicion remains high, prophylactic or therapeutic dose LMWH should be considered for the remainder of the pregnancy and for 6 weeks postpartum, depending on the level of clinical suspicion.
Magnetic resonance imaging (MRI) should be considered to diagnose iliac DVT, where the diagnosis may be suggested by back pain and swelling of the entire lower limb. Torkzad et al. found that approximately 10 % of pregnant women with proximal DVT examined at an average gestational age of 29 weeks had iliac vein thrombosis detected on MRI that was missed on ultrasound [36]. Based on a review of published literature on the biologic effects and safety of MRI in the obstetric patient, the Society of Obstetricians and Gynecologists of Canada concluded that fetal MR imaging is safe at 3.0 T (the unit used to denote the field strength of the magnet used for MRI – the majority of systems operate at 1.5 T) or less during the second and third trimesters, and inadvertent exposure to MRI during the first trimester has not been associated with any long-term sequelae [37]. The European Society of Uroradiology Contrast Medium Safety Committee (ESUR CMSC) recommendations on the use of gadolinium contrast media in pregnant and lactating women state: (a) pregnant women: the highest risk gadolinium contrast media are contraindicated in pregnant women. The intermediate and lowest risk gadolinium contrast media may be given to pregnant women in the lowest dose required to provide essential diagnostic information; (b) lactating women: lactating women who receive the highest risk gadolinium contrast media should stop breast-feeding for 24 h and discard the expressed milk. Lactating women who receive the intermediate and lowest risk agents should discuss with their doctor whether to discontinue breast-feeding for 24 h [38, 39].
5.4.2 Diagnosis of Pulmonary Embolism
Where there is clinical suspicion of acute PE, a chest X-ray should be performed. Although the chest X-ray is normal in over 50 % of pregnant women in whom PE is objectively diagnosed, the chest X-ray may show features of PE which include atelectasis, effusion, focal opacities, regional oligemia, or pulmonary edema.
If the chest X-ray is normal or consistent with a diagnosis of PE, bilateral whole lower limb duplex or compression ultrasound scanning should be performed. If this confirms a DVT, further imaging of the chest (and therefore the extra radiation to mother and fetus) may be avoided; the rationale is that the treatment of DVT and PE would be the same, so diagnosing or excluding PE appears to be unnecessary. If the chest X-ray is normal and the lower limb imaging is negative but the clinical suspicion remains high, a computed tomography pulmonary angiogram (CTPA) or ventilation/perfusion (V/Q) lung scan should be performed.
The chest X-ray may identify an alternative diagnosis such as pneumonia, pneumothorax or lobar collapse. If the X-ray is abnormal with a high clinical suspicion of PE, CTPA should be performed. If the CTPA or V/Q is also normal but the clinical suspicion of PE remains, either prophylactic or therapeutic dose LMWH should be given for the remainder of the pregnancy, depending on the level of clinical suspicion.
The guidance summarized above is broadly in line with the Royal College of Obstetricians and Gynaecologists’ (RCOG) green top guidelines [21]. Figure 5.1 shows a suggested algorithm for suspected non-high risk PE (i.e. without shock, hypotension, signs of pulmonary hypertension) in pregnancy and the puerperium.
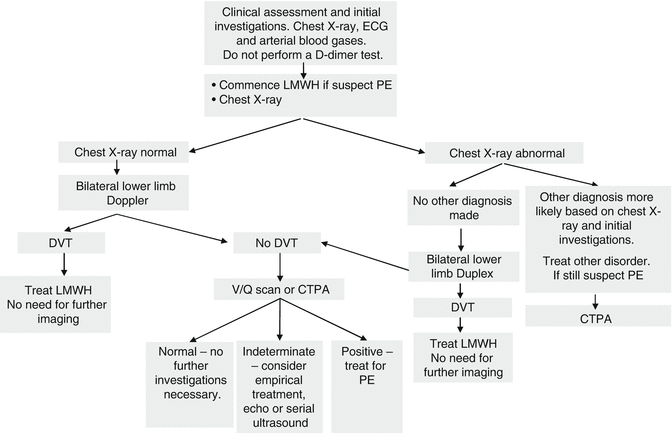

Fig. 5.1
Diagnostic algorithm for suspected pulmonary embolism in hemodynamically stable women (i.e. without shock, hypotension, signs of pulmonary hypertension) during pregnancy and the puerperium. 1) Investigations should be completed within 24 h of admission with suspected PE, 2) Chest X-ray signs include small effusion, prominent pulmonary vasculature, regional oligemia, and 3) Depending on clinical scenario, consider intervention: temporary IVC filter/thrombolysis/thrombectomy; discuss with local experts
Initial investigations should include arterial blood gases and an electrocardiogram (ECG). Oxygen saturation measured with a pulse oximeter may show resting hypoxia. Arterial blood gases (ABG) usually show a reduced PaO2 and normal or low PaCO2. The ECG may be normal except for a sinus tachycardia. Large emboli may lead to features of acute right heart strain (right axis deviation, right bundle branch block, peaked P waves in lead II); the classical S1, Q3, T3 pattern is rare. Electrocardiography is also useful in excluding other diagnoses such as acute myocardial infarction and pericardial disease.
5.4.2.1 Lung Imaging in Pregnancy and the Puerperium and Associated Radiation Risks
The dose of radiation received by the fetus from a plain chest X-ray is negligible (0.2 mGy), particularly if lead screening is used. The National Council of Radiation Protection and Measurements in the USA states that the risk (of abnormality) is considered to be negligible at 50 mGy or less when compared to other risks of pregnancy [40, 41].
The choice between CTPA and V/Q scanning for the diagnosis of PE in pregnancy is controversial. Both CTPA and V/Q scanning have been reported to have high negative predictive value, albeit in retrospective studies [42, 43]; each has its advantages and disadvantages [21]. One advantage of CTPA is that the dose of radiation to the fetus is generally lower than that associated with a V/Q scan: 0.03–0.66 versus 0.32–0.74 mGy, respectively; The wide range of values reflects heterogeneity in the protocols and equipment used as well as differences in size and age of the fetus at the time of exposure [41]. Pregnant breast tissue is particularly sensitive to radiation and every effort should be made to minimize radiation exposure during pregnancy. Some authors recommend V/Q scan as the first-line investigation in pregnancy, as it has a high negative predictive value, lower radiation dose to the pregnant breast, and because most pregnant women in the UK will not have co-morbid pulmonary pathology [44]. If the initial chest X-ray is normal, the ventilation portion of the V/Q scan may be omitted; the perfusion scan alone will often be enough to confirm or exclude PE. Iodinated contrast medium used with CTPA has the potential to affect fetal and neonatal thyroid function, although a retrospective study of 344 pregnant women who underwent a CTPA for suspected PE found normal thyroxine levels in all neonates at the time of birth [45]. Technetium-99, which is used for perfusion scans, is excreted in the urine and secreted into breast milk. The fetal radiation dose is higher and as the contrast is often concentrated in the bladder near the fetal head.
The choice of optimal lung imaging is still debated and will be decided to some extent by local availability. The RCOG recommends that “where feasible, women should be involved in the decision to undergo CTPA or V/Q scanning. Ideally, informed consent should be obtained before these tests are undertaken” [21].
5.4.2.2 Lung Imaging in Pregnant Patients: Practice and Counseling Points
All investigations should be discussed with the patient and this should be documented in her clinical records.
It is not clear what the absolute radiation risks are for fetus or mother; but they are small compared to the risk of a missed diagnosis of PE.
The discussion with the patient should include an explanation of the risks of undiagnosed PE.
Treatment of VTE with LMWH, whilst relatively safe in pregnancy, carries a risk of anticoagulant-induced bleeding. This risk is acceptable when treating a proven VTE.
The radiation dose from a chest X-ray is very small. The fetus is shielded with a lead apron.
A Consultant Radiologist should be involved in any imaging in a pregnant patient to ensure that the scanning process is optimized, in order to improve the diagnostic yield.
The radiation dose to the fetus is lower in a CTPA than in a V/Q scan. However, there is a greater radiation dose to the proliferating breast tissue. The current estimated lifetime risk of breast cancer is 1 in 8 [46]. Whether or not this small amount of extra radiation translates to a measurable increase in the lifetime risk of breast cancer is not yet known [47].
The RCOG guidelines state that women with suspected PE should be advised that, compared with CTPA, V/Q scanning may carry a slightly increased risk of childhood cancer but is associated with a lower risk of maternal breast cancer; in both situations, the absolute risk is very small [21].
The algorithm suggested uses lower limb scanning initially as evidence of VTE. If the patient has symptoms suggestive of PE but the lower limb Doppler/Duplex scan is negative, then CTPA or V/Q scanning should be performed, as the benefit of having direct lung imaging would outweigh risks of missed PE. CTPA also has the benefit of demonstrating other lung pathology that may cause symptoms, e.g. pneumonia, pleural effusion.
Ventilation perfusion (V/Q) scanning can be considered for women in whom CTPA is contraindicated, i.e. contrast allergy, renal failure. Technetium-99 is used in the perfusion scan and is excreted in the urine and breast milk. Compared to CTPA, there is less radiation to the maternal breast. However, the fetal radiation dose is often higher as the contrast is concentrated in the mother’s bladder near the fetal head. Women undergoing a V/Q scan should be well hydrated and encouraged to empty the bladder frequently. To follow the principle of keeping exposure as low as possible, it is reasonable to express and discard breast milk for 12 h after a V/Q scan. Neonatal thyroid testing is recommended after CTPA scanning using iodinated contrast medium and V/Q scanning [38].
5.4.3 Diagnosis of Cerebral Vein and Venous Sinus Thrombosis
Neuroimaging is the most important method for diagnosing cerebral vein or venous sinus thrombosis (CVT), which requires the clear demonstration of absence of flow and intraluminal venous thrombus by CT or MRI. CT venography, MR venography, blood-sensitive MRI sequences (e.g. gradient echo T2* or susceptibility weighted imaging) are useful techniques. Formal digital subtraction angiography may occasionally be needed in uncertain cases. Isolated thrombosis of small cortical veins may be challenging to detect. All cases must be discussed with a neurologist and neuroradiologist to ensure that the most appropriate tests are performed, and are interpreted correctly.
5.4.4 D-dimer
Measurement of D-dimer is currently not recommended in the investigation of suspected acute VTE in pregnancy. This is because D-dimer levels may be raised in normal pregnancy, particularly in the third trimester and in the puerperium. They are also increased in other pregnancy-related pathologies such as preeclampsia [21]. However, Chan et al. reported that by using higher cut-off points than those used in non-pregnant patients, the specificity of D-dimer assays for the diagnosis of DVT in pregnancy can be improved without compromising sensitivity, and recommended validation in prospective management studies [48]. Murphy et al. reported in healthy pregnant women attending for routine antenatal care, that there is a continuous increase in D-dimer levels with increasing gestation, and constructed a 95th percentile cutoff [49]. Although promising, the role of D-dimer levels in the diagnosis of pregnancy-associated VTE is not established.
5.5 Management of Acute Venous Thromboembolism
The optimal management of acute VTE requires a multidisciplinary approach, including senior obstetric, hematological and anesthetic input, liaison with the hemostasis laboratory, and additional input from a respiratory physician and neurologist in the case of suspected PE or suspected CVT, respectively.
As soon as the possibility of acute VTE is suspected, treatment dose LMWH should be started and appropriate investigations arranged. When the diagnosis is objectively confirmed, the aim of anticoagulation therapy in patients with established DVT is to prevent extension and reduce the risk of recurrence; in patients with established PE, the aim is to limit extension and prevent death [9]. It is important to realize that, while there is good (level 1) evidence guiding the treatment of acute VTE in non-pregnant patients, there is by comparison remarkably little good quality evidence in pregnancy. As a result, guidelines relating to pregnant women are generally extrapolated from the non-pregnant population [50–53].
The management of acute VTE will be primarily medical, that is, the use of anticoagulants, but non-medical therapies must not be forgotten. Reversible risk factors for VTE, for example hyperemesis, wherever possible should be corrected, to enable optimal management.
5.5.1 Pharmacological Therapies: The Options in Patients with Acute Venous Thromboembolism
The reader should refer to Chap. 2 on Anticoagulants and Antiplatelet Agents in Pregnancy. In non-pregnant patients, therapeutic options for VTE include unfractionated heparin (UFH), LMWH, and coumarins.
LMWH is now widely used for prophylaxis and treatment of maternal thromboembolism. The change in practice from the use of UFH is based largely on the results of large trials in non-pregnant patients that show that LMWHs are at least as safe and effective as UFH for the treatment of VTE [54, 55]. Accordingly, the American College of Chest Physicians’ (ACCP) guidelines recommend LMWH, rather than UFH, for the prevention and treatment of VTE in pregnant patients [56]. In the UK and Ireland, over 95 % of centers use LMWH to treat acute VTE in pregnancy [57]. The only exception is massive PE with cardiovascular compromise, when thrombolysis should be considered, and in this instance initial UFH is preferable because of its rapid onset of action and short half-life (see below).
Coumarin derivatives such as warfarin cross the placenta; if taken in early pregnancy (6–12 weeks of gestation), they can cause a typical embryopathy, and later in pregnancy may cause microcephaly, probably secondary to small fetal cerebral hemorrhages. There is also a risk of major fetal/neonatal intracerebral hemorrhage if the woman (and therefore the fetus) is anticoagulated around the time of delivery. Consequently, coumarins are avoided in pregnancy although they are an option in the highest-risk women, principally those with a metallic heart valve.
Newer oral anticoagulants, such as dabigatran, rivaroxaban, apixaban and edoxaban, are
Contraindicated in pregnancy. Fondaparinux and hirudin have not yet had their safety adequately
Established in pregnancy, and both cross the placenta, with the fondaparinux level in cord blood
Documented to be approximately 1/10 of the level in the maternal plasma [58]. Low dose aspirin
Is safe in pregnancy [56] but it is not effective for the treatment for VTE.
5.5.1.1 Unfractionated Heparin
Heparin is a natural product obtained from bovine or porcine mucosa. Naturally occurring heparin is comprised of a mix of molecules with differing molecular weights (range 5,000–35,000 Da). Only a third of these molecules contain the high-affinity pentasaccharide that mediates the primary anticoagulant effect of heparin. This accelerates its inhibition of thrombin (factor IIa) and factor Xa. Because of its large molecular weight, high degree of ionization, and poor lipid solubility, UFH does not cross the placenta and is therefore safe (from the fetal point of view) to use in pregnancy [59, 60]. Neither is it secreted into breast milk, so it is also safe to use in breast-feeding women. Heparin is cleared firstly by depolymerization after binding to macrophage receptors; this phase is rapid and can quickly become saturated. Heparin is then cleared by a much slower renal mechanism. When using concentrations of UFH in the therapeutic range, most is cleared by the rapid saturable mechanism; as a result, further rises in dosage can have a nonlinear effect on the anticoagulant effect. In general, therapeutic doses of UFH have a half-life of less than 60 min [61].
The effect of UFH is generally monitored using the APTT, with a target range for the APTT ratio of 1.5–2.5 (although each laboratory should derive its own APTT ratio based on anti-Xa levels). In pregnancy, there is increased binding of UFH to plasma proteins and increased concentrations of factor VIII and fibrinogen; as a result, pregnant women require higher doses of UFH to achieve the same prolongation of APTT. As a result of the heterogeneous mix of molecules in heparin, the clearance mechanisms discussed above, and the variable binding of heparin to plasma proteins, adjusting the dose of UFH in order to keep the APTT ratio in the therapeutic range can be problematic. Specific nomograms and, if necessary, anti-factor-Xa assays should be used (with a target anti-Xa range of 0.35–0.70 units/mL).
If prolonged therapy with UFH is used, the platelet count should be monitored from days 4–14. Heparin induced thrombocytopenia (HIT) should be suspected if the platelet count falls by 50 % or more and/or the patient develops new thrombosis or skin allergy between days 4 and 14 of heparin administration [62, 63]; in this situation, urgent hematological advice should be sought.
Protamine sulfate rapidly reverses the anticoagulant effect of UFH. The dose of protamine is determined by the heparin exposure: 1 mg of protamine neutralises 80–100 IU of UFH when administered within 15 min of the heparin dose. Less is required if protamine is given after a longer period because of the short half-life of intravenous UFH. Caution should be exercised with higher doses because of a paradoxical risk of bleeding.
5.5.1.2 Low-Molecular-Weight Heparin
Low-molecular-weight heparins are produced synthetically by cleaving UFH either enzymatically (e.g. tinzaparin) or chemically (e.g. dalteparin, enoxaparin). This process yields LMWHs which have a molecular weight around 4,300–5,000 Da, around a third the molecular weight of UFH. Both LMWH and UFH bind to antithrombin, facilitating its anticoagulant effect. However, in all LMWHs the anti-Xa activity exceeds the anti-IIa activity.
Because LMWH has far less interaction with acute-phase proteins in the blood and because its clearance is primarily by the renal route, its pharmacokinetic characteristics are quite different to those of UFH and its anticoagulant activity much more predictable. This means that, particularly in non-pregnant patients, fixed weight-adjusted dosages can be used without the need for laboratory monitoring. However, in pregnancy, weight changes with gestation, the volume of distribution of the drug increases significantly and there is a rise in renal clearance. Glomerular filtration rate increases significantly in pregnancy, resulting in more rapid renal clearance of LMWH, decreasing its half-life [64–66]. Consequently, the ACCP recommends a twice-daily therapeutic dosage regimen for LMWHs in the treatment of VTE in pregnancy, in contrast to the once-daily regimens used in non-pregnant patients [56]. A study of pharmacokinetics of enoxaparin during the antenatal period in 123 women suggests that, for enoxaparin, once daily dosing may suffice [67]. However, the optimal dosing strategy can be substantiated only with published clinical outcome data on safety and efficacy. The RCOG guidelines state that “there is insufficient evidence to recommend whether the dose of LMWH should be given once daily or in two divided doses” [21].
In non-pregnant subjects, LMWH has been shown to be at least as effective as UFH [54]; in one meta-analysis, LMWH was more effective than UFH in the initial management of VTE [68]. In addition, LMWH and UFH use compare favorably to oral anticoagulation with warfarin [53, 69, 70]. Systematic reviews have shown that LMWH is safe in pregnancy and does not cross the placenta [60, 71]. LMWHs are also much less likely than UFH to cause HIT [72] or osteoporosis [60, 73–78]. In a systematic review of 2,777 pregnancies treated for acute VTE, the use of LMWH was safe and effective, being associated with a recurrence rate of VTE of just 1.15 % [60]. Similar studies in non-pregnant women using either LMWH or UFH followed by coumarins reported a VTE recurrence rate of between 5 and 8 % up to 6 months after the initial event. Interestingly, in the 2,777 pregnancies treated with LMWH, not a single case of HIT was reported. The risk of heparin-induced osteoporosis also appears to be significantly lower with LMWH compared with UFH [35, 60, 74, 76–78]. Although clearly more data are needed, the risk of this complication appears very low, in the order of 0.04 %. In spite of this apparently low incidence, it seems reasonable to encourage these women to take vitamin D supplementation if the vitamin D level is suboptimal, together with calcium if the calcium is low or low normal.
Although rare, skin reactions to LMWH are well recognized [60, 79]. These may be due to type 1 immediate hypersensitivity reaction or type 4 delayed hypersensitivity reaction. When they occur, treatment with the LMWH preparation being used should be discontinued and an alternative LMWH preparation substituted. If a skin reaction occurs, HIT should be considered, although this is most unlikely with LMWH [60, 62, 63]. If LMWH is not tolerated, options for therapeutic anticoagulation include vitamin K antagonists, UFH, or fondaparinux (although experience in pregnancy with fondaparinux is limited).
Protamine sulfate incompletely reverses (by approximately 60 %) the effect of LMWH based on data from healthy human volunteers [80], although the limited data available suggest clinical benefit. Van Veen et al. described three patients requiring emergency surgery and 14 patients that were actively bleeding whilst receiving LMWH and who received protamine at doses suggested by the ACCP guidelines [81]. Protamine prevented excessive bleeding in all the surgical patients and was effective in 8 of 12 evaluable patients with active bleeding. Anti-Xa levels after protamine sulfate administration did not correlate with the likelihood of persistent bleeding [82]. The BCSH guideline on the management of bleeding in patients on antithrombotic agents recommends the following: (a) LMWH administration within 8 h of the time of requirement for correction of anticoagulation: give protamine sulfate (1 mg per 100 anti-Xa units of LMWH); (b) if ineffective, consider further protamine sulfate 0.5 mg per 100 anti-Xa units (2C). Protamine sulfate should be given more slowly than 5 mg/min to minimize the risk of adverse reactions; (c) LMWH administration more than 8 h from the time of requirement for correction of anticoagulation: consider smaller doses of protamine (2C); and (d) consider recombinant factor VIIa (rFVIIa) if there is continued life-threatening bleeding despite protamine sulfate and the time frame suggests there is residual effect from the LMWH contributing to bleeding (2C) [83].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree