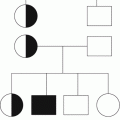
Preexisting
Previous VTE
Single
Estrogen or pregnancy related
Thrombophilia related
Unprovoked
Related to temporary risk factor
Recurrent events
Thrombophilia
Heritable
Antithrombin deficiency
Protein C deficiency
Protein S deficiency
Factor V Leiden
Prothrombin gene G20210A
Acquired
Antiphospholipid syndrome – persistent lupus anticoagulant or persistent moderate/high-titre anticardiolipin or anti-β2-glycoprotein 1 antibodies
Medical comorbidities including
SLE
Nephrotic syndrome
Heart disease
Sickle cell disease
Cancer
Inflammatory conditions, e.g. inflammatory bowel disease
Others
Age >35 years
Obesity BMI >30 kg/m2 either prior to pregnancy or in early pregnancy
Parity ≥3
Smoking
Varicose veins: gross, symptomatic, above knee, or with phlebitis or skin changes
Paraplegia
Family history of VTE
Obstetric
Antenatal
Multiple pregnancy, assisted reproduction therapy (ART)
Preeclampsia
Delivery
Cesarean section
Prolonged labor, midcavity rotational operative delivery
Postnatal
Postpartum hemorrhage (>1 L)
Blood transfusion
New onset/transient
Early pregnancy
Hyperemesis gravidarum
Ovarian hyperstimulation syndrome
Any time in pregnancy
Surgical procedure, e.g. ERPC, appendicectomy, postpartum sterilization
Admission or immobility, e.g. symphysis pubis dysfunction
Dehydration
Systemic infection, e.g. pneumonia, pyelonephritis, wound infection
Travel of duration >4 h
4.2.1 Thrombophilia: Heritable
A heritable thrombophilia is found in 20–50 % of women with pregnancy-related VTE [3]. However, different thrombophilias have different levels of risk. In a retrospective study of 72,000 pregnancies in which women with VTE were investigated for a thrombophilic tendency and the background prevalence of these defects was known, the risk of VTE in pregnancy was estimated to be 1:2.8 in type I antithrombin deficiency (with reduced activity and antigen), 1:42 for type II antithrombin deficiency (with reduced activity and normal antigen level), 1:113 for protein C deficiency, and 1:437 for factor V Leiden [4].
4.2.1.1 Factor V Leiden and Prothrombin G20210A
The most common heritable thrombophilic tendencies in the UK are factor V Leiden and pro-thrombin F2G20210A, present in around 4 and 2 % of the population, respectively. Case– control studies show that individuals heterozygous for these genes are at roughly fivefold increased risk of VTE in both the general population and pregnancy [5–7]. However, the absolute risk appears to be small (<1 %). Cohort studies undertaken in the general population and a further study in women heterozygous for factor V Leiden recorded three episodes of VTE in 752 pregnancies [8–11]. Therefore, the benefit of thromboprophylaxis at this level of risk would be limited.
The absolute risk may be higher, however, in women with a family history of VTE and a thrombophilic genotype. In a meta-analysis [8, 12], 2 % of factor V Leiden carriers had a pregnancy-related VTE. In the cohort studies where the cases were selected because of family screening, that is, two or more first-degree relatives with VTE but exclusion of the proband from analysis, 3 % of factor V Leiden carriers had a pregnancy-related VTE compared to 0.6 % in family members who were not carriers. This absolute risk is similar in magnitude to that seen in retrospective analyses in women with pregnancy-related thrombosis and with the F2G20210A prothrombin gene variant, selected because of a family history of VTE, and to those observed in a retrospective family study in carriers of factor V Leiden and F2G20210A [13–15]. A review of heterozygous factor V Leiden carriers with at least one symptomatic first-degree relative estimated the incidence of the first episode of VTE occurring in association with pregnancy at 2.1 %. The risk during pregnancy, however, was only 0.4 % compared to 1.7 % in the postpartum period. Very similar incidences were estimated for the prothrombin variant – 0.5 % in pregnancy and 1.9 % postpartum [16].
The risk of pregnancy-related VTE also appears to increase with compound (combined defects) or homozygous states. In previously asymptomatic women, the risk is higher in compound heterozygotes for factor V Leiden and F2G20210A, with an absolute risk of approximately 4 %, although a retrospective family cohort study did not confirm this increased risk [14, 17, 18]. A systematic review suggests that women who are homozygous for factor V Leiden or F2G20210A are also at much higher risk of pregnancy-related VTE, and absolute risks of 9–16 % have been reported for homozygous factor V Leiden [11, 19].
4.2.1.2 Antithrombin, Protein C, and Protein S Deficiencies
Outside pregnancy, the risk of the first VTE appears to be higher in individuals with deficiencies of antithrombin, protein C, or protein S compared to V Leiden and the F2G20210A [20]. Asymptomatic women with protein C or protein S deficiency probably have a moderately increased risk of VTE associated with pregnancy, again with most events occurring postpartum. The risk associated with antithrombin deficiency appears to vary according to the subtype but may be associated with a very high absolute risk of 15–50 % [21].
In a more recent retrospective cohort study of women from families with hereditary antithrombin, protein C, or protein S deficiency, 12 pregnancy-related VTE episodes were objectively diagnosed in 162 pregnancies (7 %), two-thirds of which occurred in the postpartum period [22]. In a recent review, in women with a deficiency of antithrombin, protein C, or protein S and at least one symptomatic first-degree relative, the incidence of the first episode of VTE occurring in association with pregnancy was estimated at 4.1 % (1.7–8.3 %). Again, the incidence appeared to be higher during the postpartum period than during pregnancy, 3 and 1.2 %, respectively [16].
4.2.1.3 MTHFR
Homozygosity for a thermolabile variant of the gene for methylenetetrahydrofolate reductase (C677T MTHFR) is sometimes included in thrombophilia testing, but there is no evidence of an association with a clinically relevant increase in the risk of VTE in pregnancy [19].
4.2.2 Thrombophilia: Antiphospholipid Syndrome
Antiphospholipid syndrome (APS) is defined as the presence of persistently positive antiphospholipid antibodies (aPL) – lupus anticoagulant (LA) and/or anticardiolipin (aCL) and/or anti-β2-glycoprotein 1 (β2 GP1) antibodies of medium or high titre on two consecutive occasions at least 12 weeks apart (“persistently positive”) in association with a history of arterial or venous thrombosis or adverse pregnancy outcome. An adverse pregnancy outcome is defined as either a fetal death after 10 weeks’ gestation, a preterm birth at less than 34 weeks’ gestation due to severe preeclampsia or intrauterine growth restriction, or the occurrence of three or more unexplained miscarriages before 10 weeks’ gestation [23].
Antiphospholipid antibodies, in particular persistent LA or moderate/high-titre aCL antibodies or a β2 GP1 antibodies, are associated with an increased risk of recurrent thrombosis, and it is common for such women to be on long-term warfarin after a first event [24–26].
Optimal management for the prevention of recurrent thrombosis in pregnancy is unclear. There is a lack of randomized trials and very few prospective studies of women with APS and prior thrombosis. In one study, 98 of 565 women tested were found to have positive aPL and were divided into low, high, or very high risk based on their clinical history, that is, asymptomatic, three or more pregnancy losses, or prior venous or arterial thrombosis, respectively [27]. Women who were low risk received 2 weeks of postpartum LMWH and antenatal LMWH if there were additional risk factors. Women in the latter two groups received prophylactic or high-prophylactic-dose LMWH (dalteparin 50–100 and 100–200 IU/kg/day, respectively) from enrollment until 6 weeks postpartum (or until oral anticoagulation was recommenced). They also received low-dose aspirin from weeks 12 to 36. There were no VTE events in the low- or high-risk groups, but there were two events in the 28 patients in the very-high-risk group, suggesting a significant risk of thrombosis. In a further series of 33 women with primary APS, women who had no previous history of thrombosis and no other risk factors were given 3–5 days of thromboprophylaxis postpartum only, and there were no thrombotic events in this subgroup [28].
4.2.3 Previous Venous Thromboembolism
Women with previous VTE have an increased risk of recurrence in pregnancy and postpartum, with reported recurrence rates of 1.4–11.1 %, and this risk appears to be constant over the whole duration of pregnancy [29, 30]. A retrospective comparison of the recurrence rate of VTE during pregnancy and the nonpregnant period revealed recurrence rates of 10.9 % during and 3.7 % outside pregnancy, giving a relative risk during pregnancy of 3.5 (95 % CI 1.6–7.8) [31].
In order to aid risk assessment, women with previous VTE can be stratified into those with recurrent or single previous VTE. The latter group may be further subdivided into those with:
A temporary risk factor associated with the VTE, for example, major trauma or surgery
Estrogen-provoked, that is, pregnancy or estrogen-containing contraception
Unprovoked
Thrombophilia, either heritable or acquired or associated with a family history of VTE
4.2.3.1 Recurrent VTE
Individuals with recurrent VTE are at increased risk of further recurrence [32]. Many will therefore be on long-term therapeutic dose warfarin. Although data are lacking, these women would be expected to have a high risk of recurrence in pregnancy.
4.2.3.2 Temporary Risk Factor-Associated VTE
Outside pregnancy, there is a low risk of recurrence of VTE which resulted from a transient major risk factor. This is also likely to be the case in pregnancy, as both a prospective and a retrospective study suggested that the risk of antenatal recurrence is very low if the prior VTE was provoked by a transient major risk factor that is no longer present [33, 34]. Examples of this include a DVT post surgery or trauma or in an intravenous drug user who is no longer injecting.
4.2.3.3 Single Previous Estrogen Related
Although prior estrogen-provoked VTE was not found to be a risk factor for VTE in subsequent pregnancy in the study by Brill-Edwards and colleagues [33], other retrospective studies suggest the contrary [30, 31, 34]. For example, in women whose previous VTE was associated with the use of estrogen-containing contraception, the recurrence rate was 9.5 % in subsequent pregnancies where thromboprophylaxis was withheld [34]. The risk was very similar (9.8, 95 % CI 4.2–20.9 %) if the prior VTE had occurred during a previous pregnancy. In another study comparing pregnant women whose prior episode was provoked by estrogen-containing contraception and those women without a history of contraceptive use at the time of VTE, the recurrence rates were 10 and 2.7 %, respectively [30].
A retrospective study using Californian hospital discharge data analyzed recurrence rates in women with a previous single pregnancy-related VTE and in women with a previous unprovoked thromboembolic event [35]. The overall recurrence rates over the following 6–60 months were lower in the group with pregnancy-related VTE initially (5.8 % compared to 10.4 %), but the rate of recurrence in subsequent pregnancies was higher (4.5 % compared to 2.7 %). Of the recurrent events in the women who had previously had a pregnancy-related VTE, 35 % occurred in a subsequent pregnancy, compared to 8.7 % in the group with previous unprovoked VTE. Furthermore, 71 % of the recurrences were antenatal in the former group compared to 54 % in the latter. This adds further support to the stratification of women with previous estrogen-related VTE as being at high risk of VTE in subsequent pregnancy and the puerperium.
4.2.3.4 Single Previous Unprovoked
In non-pregnant populations, unprovoked VTE has been shown to be associated with an increased risk of recurrence compared to those provoked by a temporary risk factor that is no longer present [32]. A prospective study of 125 pregnant women with a single prior episode of VTE showed that 5.9 % of women with VTE that was unprovoked or associated with a thrombophilia had a recurrence, in contrast to women with a previous VTE that was associated with a temporary risk factor and no thrombophilia, in whom no recurrences were seen [33]. In this study, estrogen-provoked VTE was included as a temporary risk factor. A retrospective study of 155 pregnancies in 88 women with a previous VTE compared the recurrence rate in women with a previous unprovoked VTE who were not given thromboprophylaxis to that in women where the prior VTE was associated with a transient risk factor. The recurrence rate was 4.2 % in the former group and none in the latter [34].
In contrast, Pabinger and colleagues [31] found that the presence or absence of a temporary risk factor did not affect the risk of recurrence in a subsequent pregnancy, although again estrogen-provoked VTE was included as a temporary risk factor, which may have influenced the results.
4.2.3.5 Thrombophilia Related
Outside pregnancy, the most common heritable thrombophilias do not substantially increase the risk of recurrence after a single event. This was shown in a systematic review of prospective studies [36] where being a heterozygote carrier of factor V Leiden increased the relative risk of recurrence with an OR of 1.39 (95 % CI 1.15–1.67). Data regarding the effect of heritable thrombophilia on the risk of recurrent VTE in pregnancy are extremely sparse.
4.2.4 Obesity
Obesity is common in the non-pregnant population, and a body mass index (BMI) above 30 kg/m2 was seen in 19 % of women aged 25–34 and 25 % of women aged 35–44 years [37]. Any increase in weight above a normal BMI appears to be associated with an increased risk of VTE in pregnancy. Being overweight (BMI 25–30 kg/m2) is very common in pregnancy, with a prevalence of approximately 50 %, and this is a risk factor for pregnancy-related VTE. The risk of VTE appears to increase further with increasing obesity, although the data are limited [38]. In one study, obesity was more strongly associated with pulmonary embolism than with deep vein thrombosis, with odds ratios of 14.9 (95 % CI 3.0–74.8) and 4.4 (95 % CI 1.6–11.9), respectively [39].
Obesity has been identified as a particular concern in two reports from the UK’s Centre for Maternal and Child Enquiries (CMACE). For the triennium 2003–2005, there were 33 deaths from pulmonary embolus [40]. In the 21 cases where BMI was recorded, 12 women were obese, that is, BMI >30 kg/m2. In the report from 2006 to 2008, 30 % of mothers who died from direct causes and for whom the BMI was known were obese, as were 24 % of women who died from indirect causes (27 % overall) [1].
4.2.5 Age
Age greater than 35 years increases the risk of antenatal and postnatal VTE, with an odds ratio of 1.3 (95 % CI 1.0–1.7) compared to that of women aged 20–34 years [41]. Simpson and colleagues [42] also showed that postnatal events were significantly associated with maternal age greater than 35 years (OR 1.4, 95 % CI 1.0–2.0).
4.2.6 First-Trimester Events
The risk of VTE may increase further in the first trimester due to other complications such as hyperemesis gravidarum (OR 2.5, 95 % CI 2.0–3.2) or ovarian hyperstimulation (OR 4.3, 95 % CI 2.0–9.4) [29, 43]. Women with ovarian hyper-stimulation are at particular risk of internal jugular vein VTE [44]. There is also an increased risk of VTE associated with surgery during pregnancy, including termination of pregnancy and ectopic pregnancy. Postsurgical thromboprophylaxis is therefore advised if indicated in these circumstances, as it is after delivery.
4.2.7 Mode of Delivery
Compared with vaginal birth, Cesarean section increases the risk of postpartum VTE. In a Canadian retrospective population-based cohort study of 46,766 women who underwent a planned Cesarean section for breech presentation and 2,292,420 women who planned to deliver vaginally, an increased risk of postpartum VTE with Cesarean section was demonstrated, with an odds ratio of 2.2 (95 % CI 1.5–3.2) [45].
Compared to elective Cesarean section, emergency Cesarean further increases the risk of VTE. A Swedish study included both elective and emergency Cesarean sections and showed a relative risk of VTE of 6.7 compared to vaginal delivery [46]. A Scottish study also compared emergency with elective Cesarean section and showed that the risk of VTE was doubled following emergency [47].
RCOG guidelines were published in 1995 that recommended thromboprophylaxis after emergency Cesarean section [48]. The CEMACE reports for the subsequent four triennia show that during this period (1997–2008), there were 26 deaths from VTE following Cesarean section compared to 27 following vaginal delivery [1]. As the latter make up 70–80 % of all deliveries, this suggests that Cesarean section remains a risk factor for fatal PE despite the published guidelines on thromboprophylaxis.
4.2.8 Immobility
Immobility is known to be a risk factor for the development of VTE in non-pregnant patients, but the information available for pregnant patients is limited. One case–control study looked at antepartum immobilization, defined as strict bed rest for 1 week or more prior to delivery, in patients with a BMI of greater than 25 kg/m2. The results showed a multiplicative effect on the risk of antepartum and postpartum VTE with odds ratios of 62.3 and 40.1, respectively [43].
The UK’s National Institute for Health and Care Excellence (NICE) guideline on antenatal care [49] and the UK’s RCOG Scientific Advisory Committee Opinion Paper on air travel in pregnancy [50] state that long-haul air travel increases the risk of VTE, but the current RCOG guideline considers all long-distance travel longer than 4 h duration, and not just by air, to be a risk factor for VTE in pregnancy [2].
4.2.9 Hospital Admission
In non-pregnant medical and surgical patients, hospitalization is now recognized as a major risk factor for VTE, and it is believed that at least 25,000 deaths per year in England resulting from PE complicating hospital admission may be preventable. An independent expert working group set up by the UK Chief Medical Officer recommended that it be mandatory for all patients to be risk assessed for VTE on admission to hospital, and this report has been accepted by the Department of Health [51]. NICE guidelines on prevention of VTE in patients admitted to hospital recommend consideration of VTE prophylaxis with LMWH for women who are pregnant or have given birth within 6 weeks who are admitted to hospital and have one or more risk factors [52].
4.3 Thromboprophylaxis
4.3.1 Efficacy
Thromboprophylaxis has been shown to reduce the incidence of VTE in pregnancy. In a study of 284 pregnancies in women with a prior event, there were no recurrent events in the 87 in which thromboprophylaxis was administered compared with eight in the 197 pregnancies where it was not [30]. In a prospective cohort study in families with antithrombin, protein C, or protein S deficiency or factor V Leiden, two VTEs occurred in 28 pregnancies (7 %) in women who did not receive thromboprophylaxis, whereas there were no episodes in 43 women who did receive thromboprophylaxis [53]. Another study compared the incidence of postpartum VTE before and after the introduction of thromboprophylaxis across Scottish maternity hospitals in 1995. There were approximately 1.55 million maternities between 1980 and 2005, and there was a significant reduction in the incidence of VTE between 1996 and 2004 compared to 1980–1995 [54].
4.3.2 Pharmacological Agents
4.3.2.1 Low-Molecular-Weight Heparins (LMWHs)
LMWHs have been shown to be as effective as, and safer than, unfractionated heparin (UFH) when used to prevent VTE in pregnancy [55, 56]. UFH can cause heparin-induced thrombocytopenia (HIT), but the risk of this is much lower when LMWHs are used. Current guidelines support monitoring the platelet count in patients on LMWH only when there has been previous exposure to UFH. Prolonged use of UFH during pregnancy can result in osteoporosis and fractures, but this risk is very low with LMWH [57]. In a systematic review of prophylactic and treatment dose LMWH use in 2,777 pregnancies, there were no cases of HIT; the incidence of osteoporotic fractures was 0.04 % and of allergic skin reactions was 1.8 % [55]. Significant bleeding was seen in 1.98 % and was usually related primarily to obstetric causes.
Table 4.2 gives suggested prophylactic and therapeutic subcutaneous doses of LMWH in pregnancy and postpartum. Doses of LMWH are based on booking weight rather than BMI or weight later in pregnancy (although some authorities calculate the dose according to current weight). Data from the UK Obstetric Surveillance Study (UKOSS) show that overweight and obese women develop VTE while on prophylactic LMWH at doses appropriate for those of lower weight [58]. There is no consensus on the appropriate prophylactic dosing of obese women, so some units may prescribe the usual prophylactic dose twice daily for women over 90 kg.
Table 4.2
Suggested doses for antenatal and postnatal LMWH thromboprophylaxis
Weight | Enoxaparin | Dalteparin | Tinzaparin |
|---|---|---|---|
<50 kg | 20 mg daily | 2,500 units daily | 3,500 units daily |
50–90 kg | 40 mg daily | 5,000 units daily | 4,500 units daily |
91–130 kg | 60 mg dailya | 7,500 units dailya | 7,000 units dailya |
131–170 kg | 80 mg dailya | 10,000 units dailya | 9,000 units dailya |
>170 kg | 0.6 mg/kg/daya | 75 u/kg/daya | 75 u/kg/daya |
50–90 kg, high prophylactic | 40 mg twice daily | 5,000 units twice daily | 4,500 units twice daily |
Treatment dose (antenatal) | 1 mg/kg/twice daily | 100 u/kg/twice daily | 175 u/kg/day |
Treatment dose (postnatal) | 1.5 mg/kg/day | 200 u/kg/day | 175 u/kg/day |
It may be appropriate to use higher doses of LMWH as prophylaxis in women who are usually on long-term oral anticoagulation because of previous recurrent VTE or thrombophilia. Anti-Xa level monitoring may also be helpful in guiding therapy in women at very high risk because of antithrombin deficiency, but this should be done in association with an expert in hemostasis.
4.3.2.2 Unfractionated Heparin
UFH has a shorter half-life than LMWH, and there is more complete reversal of its activity by protamine sulfate. UFH has several disadvantages compared to LMWH. In addition to the association with HIT and osteoporosis, when used for thromboprophylaxis, more frequent administration is required due to its shorter half-life [59].
In some women, for example those at very high risk of thrombosis or increased risk of hemorrhage, UFH may be preferred around the time of delivery so that use of regional anesthesia or analgesia is not prevented. For example, if no LMWH has been given for 24 h but the woman has not yet delivered and there is concern about delaying further doses of LMWH, a prophylactic dose of 5,000 units subcutaneously of UFH could be used and repeated every 12 h until LMWH can be safely resumed after delivery. The required interval between a prophylactic dose of UFH and regional analgesia or anesthesia is less than with LMWH (4 and 12 h, respectively), and there is less concern regarding neuraxial hematoma with UFH [60].
4.3.2.3 Danaparoid
Danaparoid, a heparinoid with a half-life of approximately 24 h, is used mostly in patients intolerant of heparin. A recent review of the use of prophylactic and treatment doses of danaparoid in pregnant women with (current or a history of) HIT or skin allergy to heparin (32 and 19 cases, respectively) showed four maternal bleeding events, two of which were fatal due to placental problems (previa and abruption) [61]. In three lactating women with measurable plasma levels, no anti-Xa activity was detected in the cord blood of five infants tested, and no anti-Xa activity was found in breast milk. There were no adverse fetal outcomes attributed to danaparoid. Use of this agent should be managed in conjunction with a consultant hematologist with expertise in this area.
4.3.2.4 Fondaparinux
Fondaparinux is a synthetic indirect factor Xa inhibitor, licensed in the UK for the prevention and treatment of VTE outside pregnancy, and is similar in efficacy to LMWH [62]. No placental transfer of fondaparinux was found in a human cotyledon model [63], but anti-Xa activity of approximately 10 % of that in maternal plasma was found in the umbilical cord plasma in newborns of five mothers being treated with fondaparinux [64]. There is limited experience of its use in pregnancy, where it may be useful in women with a history of HIT. Although no adverse effects were observed in the newborns, it is premature to conclude that it is safe, and its use should be reserved for women intolerant of heparin compounds. The regular prophylactic dose is 2.5 mg subcutaneously daily, and it does not seem necessary to alter this dose in pregnancy [65].
It is unknown whether fondaparinux is excreted in breast milk and, although oral absorption seems unlikely, its use in the postpartum setting is not currently advised.
4.3.2.5 Low Dose Aspirin
There are no controlled trials on the use of aspirin for thromboprophylaxis in pregnancy, so conclusions about its efficacy have been extrapolated from other trials in the non-pregnant population. A meta-analysis of trials of short-term antiplatelet therapy in surgical and medical patients showed a significant reduction in both DVT and PE with antiplatelet prophylaxis [66]. Another meta-analysis, focusing on patients at high risk for occlusive vascular events, found a statistically significant 25 % reduction in the odds of pulmonary embolism associated with antiplatelet therapy [67]. Another trial, much criticized, suggested that, compared to placebo, low dose aspirin reduces by 36 % the risk of VTE after orthopedic surgery, even in some patients taking concomitant heparin therapy [68]. The Women’s Health Study, however, found aspirin to be no better than placebo for long-term primary prevention of VTE in older women in a secondary end-point analysis [69]. The American College of Chest Physicians’ guidelines recommend against the use of aspirin for VTE prophylaxis in any patient group [70, 71].
No adverse fetal outcomes were reported in the meta-analyses of large randomized controlled trials of low dose aspirin in pregnancy for the prevention of preeclampsia [72]. Use of low dose aspirin is appropriate for women with APS to improve fetal outcome [73] and as conjunctive therapy when LMWH is used in pregnant women with mechanical heart valves.
4.3.2.6 Warfarin
Warfarin use in pregnancy is restricted to a few situations where heparin is considered unsuitable, that is, in some patients with mechanical heart valves. It is therefore generally not used for thromboprophylaxis antenatally.
Warfarin can be safely used following delivery and in breastfeeding mothers, although it requires close monitoring and visits to an anticoagulant clinic and, compared with LMWH, carries an increased risk of postpartum hemorrhage and perineal hematoma. It is appropriate for those on maintenance warfarin outside pregnancy to restart postnatally, but this should be delayed for at least 5–7 days after delivery to minimize the risk of hemorrhage during the period of overlap of LMWH and warfarin. It is not appropriate for those women who require short-term postpartum prophylaxis, for example, 7 days.
4.3.2.7 Dextran
Dextran should be avoided antenatally and intrapartum, primarily because of the risk of anaphylactoid reaction, which has been associated with uterine hypertonus, fetal distress, fetal neurological abnormalities, and death [74, 75]. As there are now many alternatives, dextran is of little value in modern obstetric practice.
4.3.2.8 Oral Thrombin and Xa Inhibitors
Dabigatran and rivaroxaban are licensed for the prevention of VTE after major orthopedic surgery and the latter is now licensed for treatment of acute DVT. They are not licensed for use in pregnancy where there is no experience in their use and thus should be avoided.
4.3.3 Contraindications to Pharmacological Thromboprophylaxis
LMWH should be avoided, discontinued, or postponed in women who are at risk of bleeding, after careful consideration of the balance of risks of bleeding and thrombosis. Nonpregnancy-related risk factors for bleeding in pregnancy are extrapolated from data obtained from non-pregnant populations.
Women with major antepartum hemorrhage, progressive wound hematoma, suspected intra-abdominal bleeding, and postpartum hemorrhage are at high risk of further hemorrhage and may be more appropriately managed with UFH or anti-embolism stockings. If a woman develops a hemorrhagic problem while on LMWH, the treatment should be stopped and expert hematological advice sought. Excessive blood loss and blood transfusion are also risk factors for VTE, so thromboprophylaxis should be begun or reinstituted as soon as the immediate risk of hemorrhage is reduced, again emphasizing the need for repeated review of the individual patient [43, 76].
Renal impairment is not an absolute contraindication to LMWH use, but a dose reduction is required in severe renal impairment (because of the risk of accumulation of LMWH and the association of platelet dysfunction with uremia). The dose reduction depends on the specific LMWH. For example, the dose of enoxaparin and dalteparin should be reduced in patients with creatinine clearance less than 30 mL/min, but the dose of tinzaparin should be reduced if the creatinine clearance is less than 20 mL/min. Table 4.3 summarises risk factors for bleeding.
Table 4.3




Risk factors for bleeding
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree