Introduction
As the reader will see in this chapter, systemic treatment for all stages of breast cancer is complex and rapidly changing. There are many “approved” therapies that can be submitted to insurance companies for approval in determining treatment for a given patient. One of the reasons why our group has made the decision to follow pathways in the selection of treatments for cancer patients is to prioritize clinical trials (to gain new knowledge) and to offer patients the best evidence-based care. As a group, we have been following the Elsevier ClinicalPath system, formerly known as Via Oncology Pathways. After reviewing different pathways programs, this system was selected for several reasons:
- •
The evidence-based pathways were prioritized and selected by practicing community and academic oncologists.
- •
Each disease-specific committee has both academic and community oncologist chairs and members.
- •
Committee meetings are held virtually every quarter for review of the pathways. Changes can be made at these meetings if new evidence is available. Urgent meetings can be held if new field-changing information is released.
- •
All of our oncologists who regularly treat patients with any given disease are encouraged to attend and actively participate in the disease-specific committees.
- •
The pathways allow clinical trials to be prioritized as the first recommendation.
- •
Pathways are ranked by efficacy (most), toxicity (lesser), and cost (least), prioritized in this order.
- •
In addition to a clinical trial option, for each stage of disease there are several “recommended” pathways from which the physician can select. They can also choose to treat “off-pathway” if they believe this is in the best interest of the patient.
Unlike guidelines, such as National Comprehensive Cancer Network where multiple recommendations are listed, we felt it was most important to have recommendations prioritized so that we could collect and evaluate the data of a large number of similarly staged patients treated the same way. This has also allowed us to offer and accrue a large number of patients to clinical trials to help move the field forward.
Oncologists in our group are encouraged to follow the recommended pathways, always offering their patients the ability to participate in clinical trials and to be involved in the decision-making process. The physicians have the opportunity to deviate from the recommended pathway if they believe there are reasons why the recommended treatment is not the best for a given patient. For most diseases, between 85% and 92% of patients are treated on pathway. This chapter is broken down into three main parts:
- 1.
Neoadjuvant therapy
- 2.
Adjuvant therapy
- 3.
Treatment of metastatic disease
Neoadjuvant Therapy for Breast Cancer
Introduction
Historical Perspective
The term neoadjuvant therapy for breast cancer is used in a setting where systemic therapy is utilized prior to definitive surgery. Historically, this treatment approach has been reserved for patients who had clinically unresectable disease, or for locally advanced patients who require a mastectomy to appropriately resect the entire tumor. For patients desiring breast conservation, neoadjuvant chemotherapy has been used to convert from mastectomy to lumpectomy for patients who achieve sufficient response. For this purpose, the treatment had been largely limited to the administration of neoadjuvant multiagent chemo therapy and has been largely limited to serve surgical outcomes.
Current Goals of Neoadjuvant Therapy
With the advent of more targeted agents, additional goals of neoadjuvant therapy have been identified. Beyond the surgical goal of transforming a patient for mastectomy to lumpectomy or from unresectable to resectable, the goal of neoadjuvant therapy is now also to quantify the tumor’s response to neoadjuvant therapy to prognosticate short and long-term outcomes, and to delineate adjuvant therapy strategies after surgery. Based on the response achieved, the best adjuvant treatment strategy can be identified with a more aggressive approach in patients who have a suboptimal response, and sparing treatment toxicity for patients with a good response.
With the growing knowledge of the efficacy of systemic therapy for breast cancer coupled with the understanding of the more diminished role of aggressive local therapy, neoadjuvant therapy is also exploring the concept of eradicating axillary disease with systemic therapy. The goal is to minimize the need for aggressive local therapies, such as axillary dissections and comprehensive radiation therapy.
In that, studying the clinical and pathologic response to neoadjuvant therapy has become a very useful clinical trial environment to assess the efficacy of a multitude of new agents, helping us recognize the neoadjuvant treatment response as a surrogate marker for long-term disease control.
This section describes the current state-of-the-art of neoadjuvant therapy in breast cancer with its complexities of patient selection; preoperative clinical, pathologic, and radiographic evaluation; categories of neoadjuvant therapy; post-treatment assessment; and subsequent optimized adjuvant management from a medical oncologic perspective. The principles of the surgical and radiation approach in the neoadjuvant setting are deferred to other specialty-specific chapters within this book.
Patient Selection for Neoadjuvant Therapy
Primary Tumor
As discussed earlier, neoadjuvant therapy was historically utilized in patients with locally advanced disease, to aid in resectability and achievement of breast conservation. In that, the clinical and radiographic staging assessments have become critical components of our preoperative assessment, and over time have become more complex and specific. Most patients will undergo preoperative mammography and ultrasonography, and many will also benefit from the application of preoperative magnetic resonance imaging (MRI) or contrast-enhanced mammography. This is largely used to optimize the surgical approach and understand the degree of local disease advancement including multifocality, skin, chest wall, and lymph node involvement. The decision whether the patient should undergo surgery first or should be subjected to neoadjuvant therapy is typically at the discretion of a multidisciplinary team including the surgeon, medical and radiation oncologists, pathologist, and of course the patient. In our institutions, these discussions are frequently and routinely held within multidisciplinary tumor conferences or clinics, with the goal of achieving a consensus recommendation for each individual patient.
Patients with locally advanced T3 or T4 breast cancers are typically ideal candidates for the approach with neoadjuvant therapy regardless of the subtype of breast cancer, as they are often not amenable to resection and much less to breast conservation upfront. Furthermore, most of those patients will benefit from systemic therapy due to the high risk of distant disease recurrence, and giving the treatment preoperatively will thus serve both purposes.
Certain patients with more limited disease (typically T1c and T2 tumors) will usually also be referred for neoadjuvant therapy, at least in part to identify patients who would benefit from additional more intensified adjuvant therapy if a complete pathologic response is not achieved. This is particularly true for human epidermal growth factor 2 (HER2) (HER2)/neu-positive and triple-negative breast cancers, who will typically be referred for systemic therapy even in the early-stage setting. These tumors are known to have a very high rate of achievement of clinical and pathologic complete responses.
The role for neoadjuvant chemotherapy in clinically limited ER-positive disease is less clear at this time, since neoadjuvant chemotherapy typically does not yield significant and much less so complete pathologic responses in this setting. For this reason, the use of neoadjuvant endocrine therapy is currently a strong area of research. This area has interestingly been accelerated during the time of the COVID-19 pandemic, in which patients were referred for neoadjuvant therapy to delay definitive surgical intervention even in early-stage disease, as many hospital centers chose to delay nonessential surgeries.
Lymph Node-Positive Disease
Axillary dissections and comprehensive axillary radiation therapy are well known to induce short- and long-term toxicities including lymphedema and restriction of arm mobility. Another goal in utilizing neoadjuvant therapy is to minimize the need for these interventions. The standard approach to patients with lymph node involvement has been surgical and radiation management; however, with the advent of neoadjuvant therapy and effective downstaging of axillary involvement, many patients can be safely managed with limited lymph node removal or sentinel lymph node biopsy alone. This is especially true for breast cancers with a more aggressive histology (HER2/neu-positive, triple-negative) and with relatively limited lymph node involvement (cN1).
HER2/Neu-Positive Disease
HER2/neu-positive disease represents a very specific histologic subtype for the consideration of neoadjuvant therapy, as it meets all discussed criteria for referral for neoadjuvant therapy including resectability, breast conservation, and lymph node downstaging. In addition, this particular subtype of breast cancer has shown exclusive sensitivity to combination HER2/neu-targeted therapy and chemotherapy, achieving high rates of complete pathologic responses, which have been used as surrogate markers for short- and long-term survival outcomes. With that, measurable responses achieved in the neoadjuvant setting can be used to prognosticate the benefit of additional adjuvant therapy, in which setting, patients with complete pathologic responses have excellent outcomes even with de-intensified adjuvant treatment options. Conversely, patients with suboptimal response to neoadjuvant therapy can be approached with more aggressive adjuvant treatment, aiding in improved long-term outcomes. This means that the neoadjuvant arena for HER2/neu-positive disease can often be utilized as a training ground to understand the patient’s biology of the tumor, and to aid in treatment selection after surgery.
Timing of Surgery
In certain circumstances, neoadjuvant therapy can be utilized to delay definitive surgical intervention. This has especially been true in our institutions during the COVID-19 pandemic, during which elective surgeries were deferred to minimize potential exposure and preserve valuable hospital resources. Furthermore, patient-specific characteristics sometimes dictate a medical delay before a patient can safely undergo an invasive procedure (for instance, thromboembolic events / recent pulmonary emboli, pregnancy, recent myocardial infarction, etc.). In both patient- and healthcare-specific settings, neoadjuvant therapy can be utilized to effectively treat the primary malignancy while safely delaying the surgery.
Preoperative Evaluation
The preoperative evaluation of a patient’s plan to undergo neoadjuvant therapy is complex and involves pathologic, radiographic, and clinical assessments.
Pathology
Understanding the patient’s histopathologic and immunologic pathology is paramount in predicting the patient’s likely response to neoadjuvant therapy. This includes assessment of the estrogen and progesterone receptor (PR) status, HER2/neu assessment, as well as the grade of the tumor. At our institutions, Ki-67 assessment is also the standard of care in the pathologic assessment of breast specimens. In some cases, preoperative assessment of molecular profiling (Oncotype, MammaPrint) can be useful in patients with ER-positive disease, particularly if it is unclear if the patient will or will not benefit from systemic chemotherapy (for instance, postmenopausal patients with ER-positive T1c or T2 tumors and cN0 or cN1 disease).
Radiology
The preoperative assessment of the clinical stage of breast cancer is paramount for the clinical and radiographic assessment of the stage of the disease, and should always include mammography and breast ultrasound. In most cases, pretreatment MRI is also routinely obtained in patients considered for neoadjuvant therapy in our institutions to aid in the assessment of local extent of disease and to assess for presence of multifocal disease, chest wall involvement, and more locally advanced lymph node involvement. This is especially true for patients who present with a lobular histology, as often mammography and ultrasound may underestimate the extent of this disease. It needs to be understood that pretreatment MRI is afflicted with a higher rate of false-positive findings and has shown to lead to a higher rate of mastectomy, including bilateral mastectomy, without apparent improvement in outcomes. With that, the benefit of pretreatment MRI needs to be juxtaposed to its potential risks and carefully evaluated with each patient.
All disease identified radiographically should undergo biopsy (primary tumor, lymph node) via fine-needle aspiration or core needle biopsy. At the time of biopsy, a radiopaque clip is placed that can be traced throughout the trajectory of therapy and will be removed surgically at the time of their definitive intervention. These clips are extremely useful, especially in patients who achieve significant or complete radiographic and pathologic responses, as they guide the surgical approach but also point the pathologist to the area within the histologic specimen previously involved by cancer.
Lymph Nodes
The assessment of lymph nodal status prior to the administration of neoadjuvant therapy is useful for surgical planning and is often used to select the appropriate neoadjuvant systemic treatment. To assess the lymph nodal status, we perform a clinical assessment as well as a comprehensive axillary ultrasound of all patients with a primary breast cancer diagnosis. As discussed earlier, most of those patients also undergo pretreatment MRI at our institutions. Any suspicious axillary lymph nodal involvement will require biopsy evaluation, at which time a radiopaque clip is placed, and the affected lymph node is targeted for surgical excision at the time of definitive surgery even in the setting of complete radiographic response. For patients with lymph node involvement proven by biopsy and with a clip in place, we require that the marked lymph node is removed at the time of definitive surgery in addition to sentinel lymph node biopsy. Prospective clinical trials have shown that the removal of the marked node will lower the false-negative rate of sentinel lymph node biopsy alone. Unfortunately, the marked node is present only about 75% of the time in a routine sentinel lymph node biopsy, and with that we favor preoperative localization of the marked lymph node, either via wire localization or using newer techniques, including Savi scout.
Neoadjuvant Systemic Therapy
Chemotherapy
Just like with any other neoadjuvant therapy, the purpose of neoadjuvant chemotherapy is to fulfill the goal of reducing the risk of distant recurrence, downstaging the primary malignancy, and providing clinical information of the tumor’s responsiveness to the chosen regimen, which may suggest additional adjuvant treatment options after surgery. Chemotherapy is typically employed in ER-positive/HER2/neu-negative disease and in triple-negative breast cancer; however, it needs to be recognized that neoadjuvant endocrine therapy is an evolving field that is rapidly gaining popularity. HER2/neu-positive breast cancers are approached with a combination of HER2/neu-targeted treatment and chemotherapy. Recent approvals of neoadjuvant immunotherapy in combination with chemotherapy have been achieved in triple-negative breast cancer. This is a testament to the fact that the neoadjuvant treatment arena is currently considered a very fertile area for research and the standard of care is rapidly changing and evolving.
This section is dedicated to the approach to patients who present with HER2/neu-negative breast cancer and for whom neoadjuvant chemotherapy is chosen.
The regimen selection for neoadjuvant chemotherapy for an estrogen receptor (ER)-positive but HER2/neu-negative breast cancer typically centers around one paradigm question: to utilize or not to utilize an anthracycline. This choice is typically informed by the results from the Anthracycline and Breast Cancer study, which evaluated anthracycline-based regimens in comparison to six cycles of Docetaxel and Cytoxan in the adjuvant setting. The detailed subgroup analysis of this study showed that the addition of an anthracycline typically only benefits patients with triple-negative disease or patients with four or more positive lymph nodes with ER-positive disease.
Accordingly, patients with limited (one to two axillary lymph nodes [ALNs]) disease and ER-positive presentation are typically approached with an anthracycline-free Docetaxel- and Cytoxan-based neoadjuvant approach; however, more locally advanced ER-positive patients do receive neoadjuvant Adriamycin, Cytoxan, and Taxol (administered either in the dose-dense fashion or for 12 weekly doses). However, the ECOG 1199 clinical trial showed that docetaxel given every 3 weeks is at least equivalent to the weekly administration of Taxol in the adjuvant setting and would be considered a very reasonable alternative to the typical weekly or dose-dense Taxol approach, especially in patients in whom toxicity considerations around Taxol are concerning.
The neoadjuvant treatment choice for triple-negative therapy has become more complex over the last few years, as the addition of carboplatin and immunotherapy have shown benefit in selected patients.
There is good evidence now that the addition of carboplatin to neoadjuvant therapy with taxanes and anthracycline-based regimens leads to improved complete pathologic response rates.
With that, there is emerging evidence that these improved response rates also translate into improved long-term outcomes including event-free survival, as seen for instance in the double-blind phase III BrighTNess trial, where the 4-year event-free survival improved from 69% to 79% with the addition of carboplatin. However, it needs to be noted that the addition of carboplatin also provides higher hematologic toxicities and higher treatment discontinuation rates. Interestingly, the addition of other chemotherapeutic agents to our backbone of Adriamycin, Cytoxan, and Taxol (for instance, gemcitabine, capecitabine, and bevacizumab) has not translated into clinically meaningful improvements and is not routinely recommended at our institutions.
The very recent addition of checkpoint inhibitor-based immunotherapy to chemotherapy in the neoadjuvant therapy for triple-negative breast cancer has shown significant improvements in desired outcomes, leading to the recent US Food and Drug Administration (FDA) approval of pembrolizumab in this setting. This is discussed in more detail below in the immunotherapy section.
HER2/neu-Targeted Therapy
The previously discussed narrow surgical indications for neoadjuvant therapy do not necessarily apply in the HER2/neu-positive setting, as the response achieved in the neoadjuvant setting significantly influences the choice for treatment after surgery. The achievement of a complete pathologic response is associated with long-term improvement in outcomes, in that it is both prognostic and predictive. In sum, administration of neoadjuvant therapy in HER2/neu-positive disease can help clarify the need for additional intensified (or de-intensified) adjuvant therapy.
In the early-stage setting, neoadjuvant HER2/neu-targeted combination therapy is still indicated for patients desiring breast conservation, who are not initially candidates due to the size of the tumor relative to the size of the breast. Furthermore, patients will benefit from the application of neoadjuvant therapy even in the setting of limited nodal extent in the axilla, in which scenario patients often achieve complete pathologic remission, sparing the need for full axillary dissection. Beyond those two indications for surgical downstaging, neoadjuvant HER2/neu-targeted combination therapy is often used to assess the need for postoperative therapy with trastuzumab emtansine (TDM1), which is only indicated if patients do not achieve complete pathologic responses.
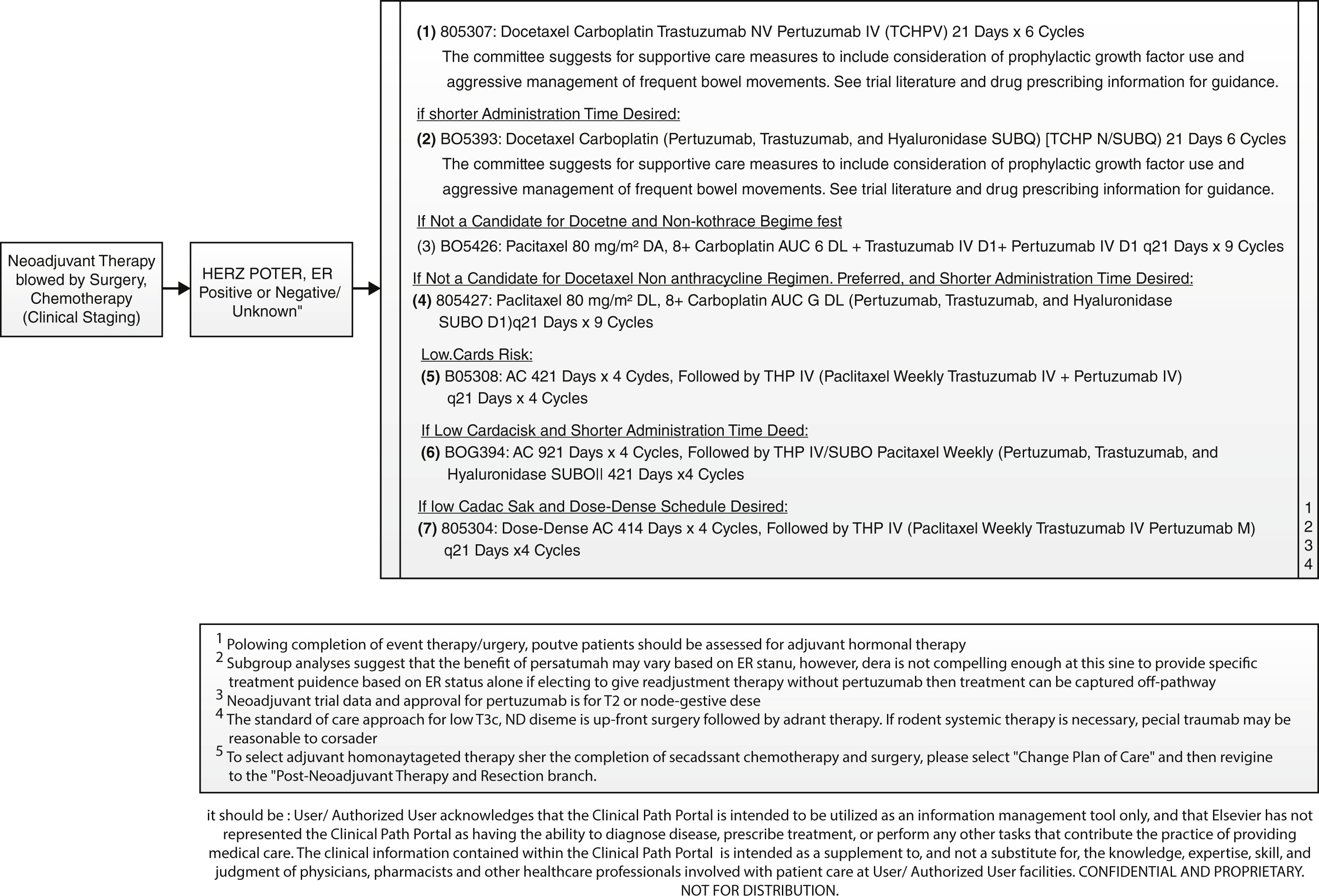
The standard neoadjuvant therapy regimen for HER2/neu-positive disease is six cycles of trastuzumab, pertuzumab, Docetaxel, and carboplatin (TCHP). Owing to the known cardiotoxicity of both monoclonal antibodies, concurrent or sequential anthracycline-based regimens have fallen out of favor, even in patients who have ER-negative disease. Due to the high toxicity afflicted with TCHP, and the high rate of achievement of complete pathologic responses, multiple clinical trials are currently ongoing that are investigating the de-escalation of this regimen. One of those trials is the COMPASS trial, which is investigating four cycles of Docetaxel, trastuzumab, and pertuzumab only, at which time patients are taken to surgery and then further adjuvant therapy is tailored to the degree of response achieved. Our institutions are participating in this trial.
Screen shots demonstrating how this regimen is selected in the EMR are shown in the following seven figures:
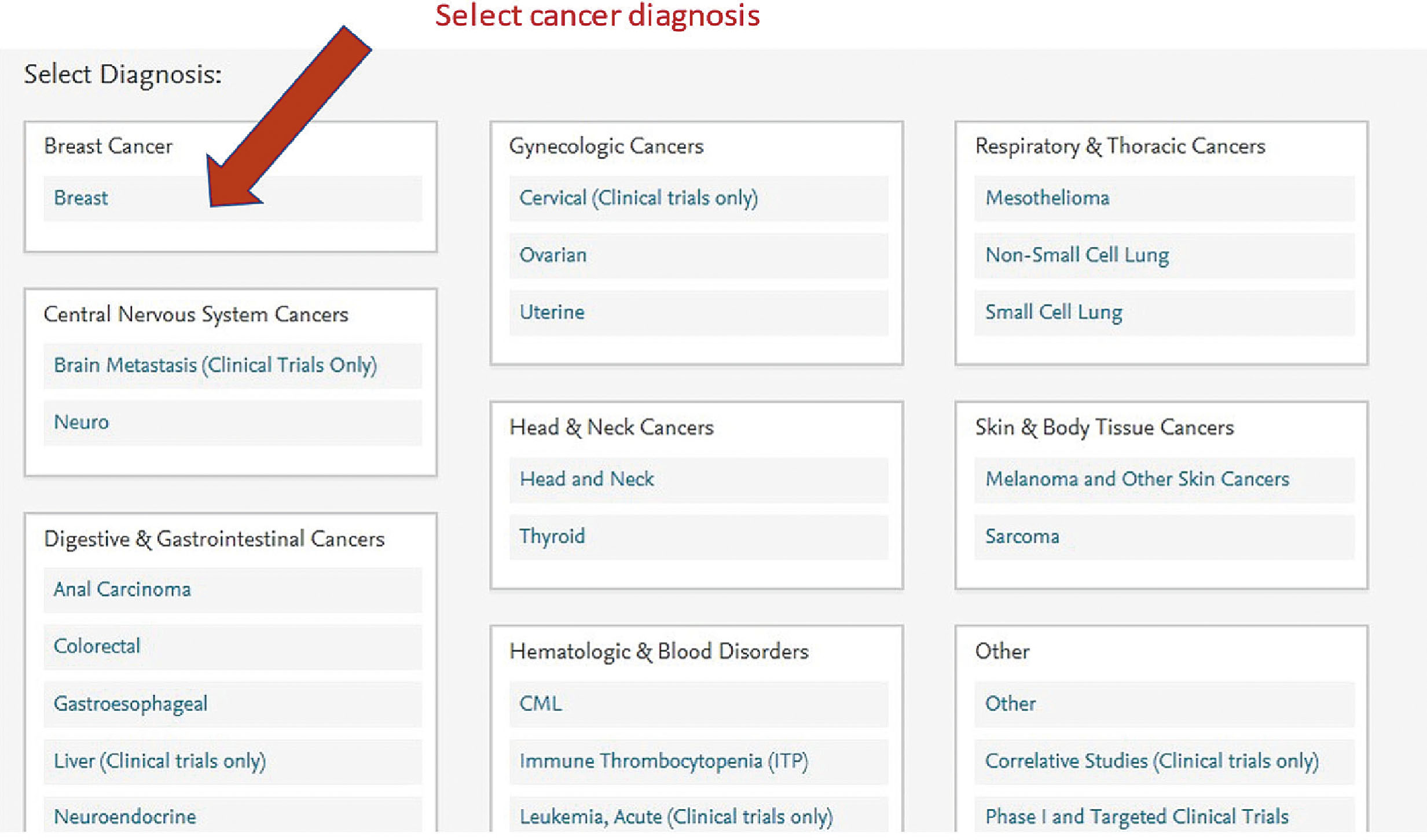

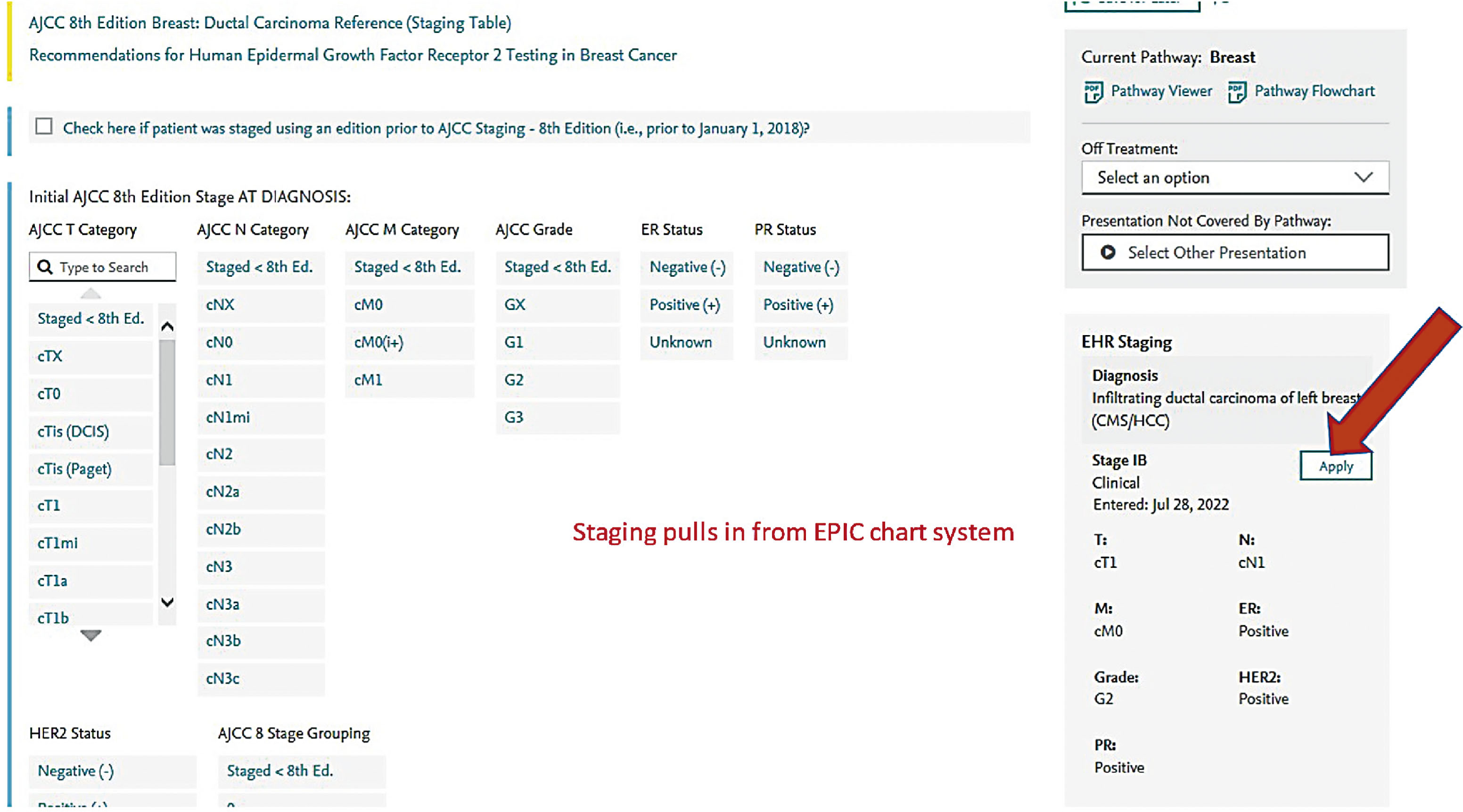
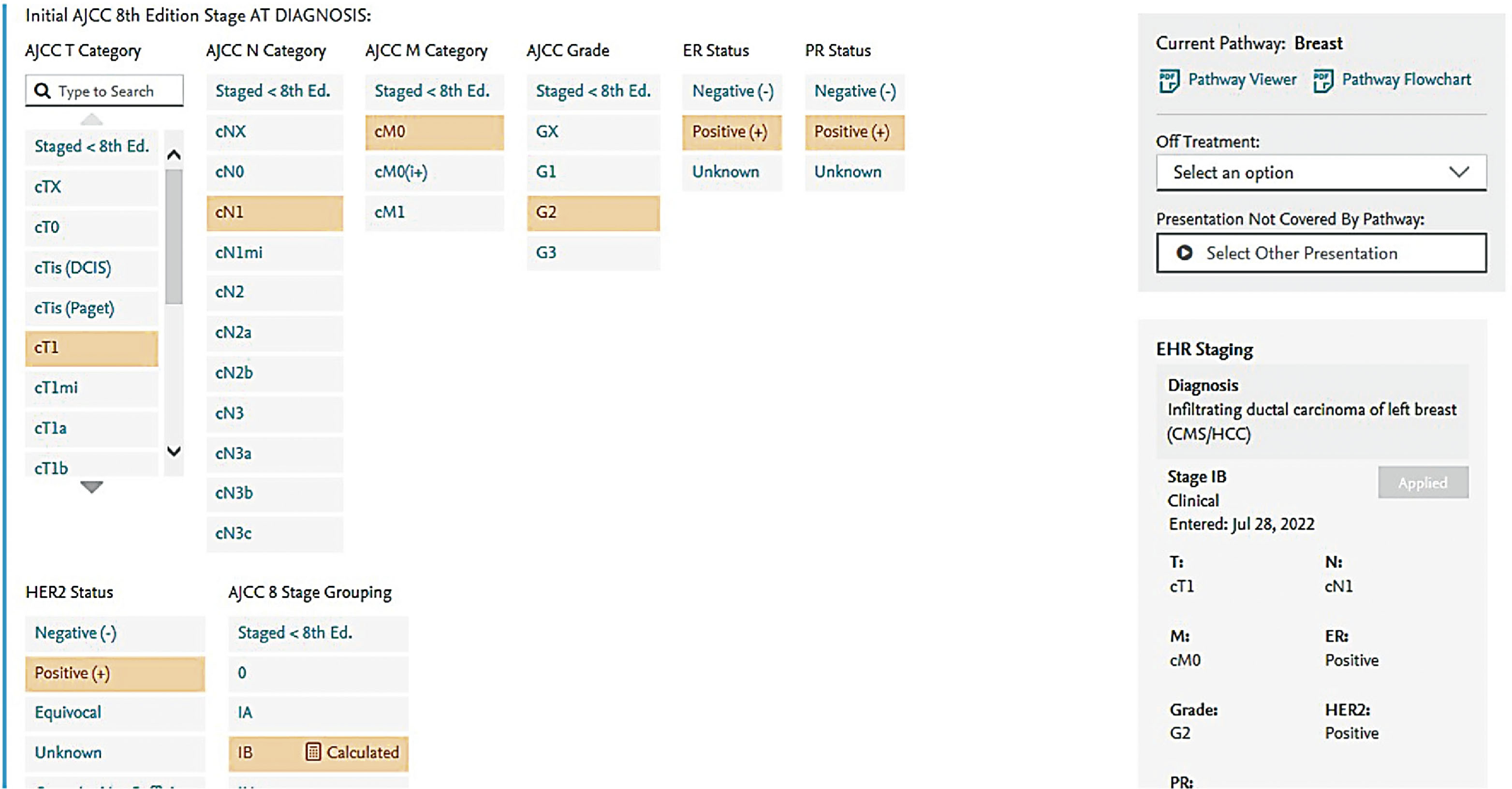
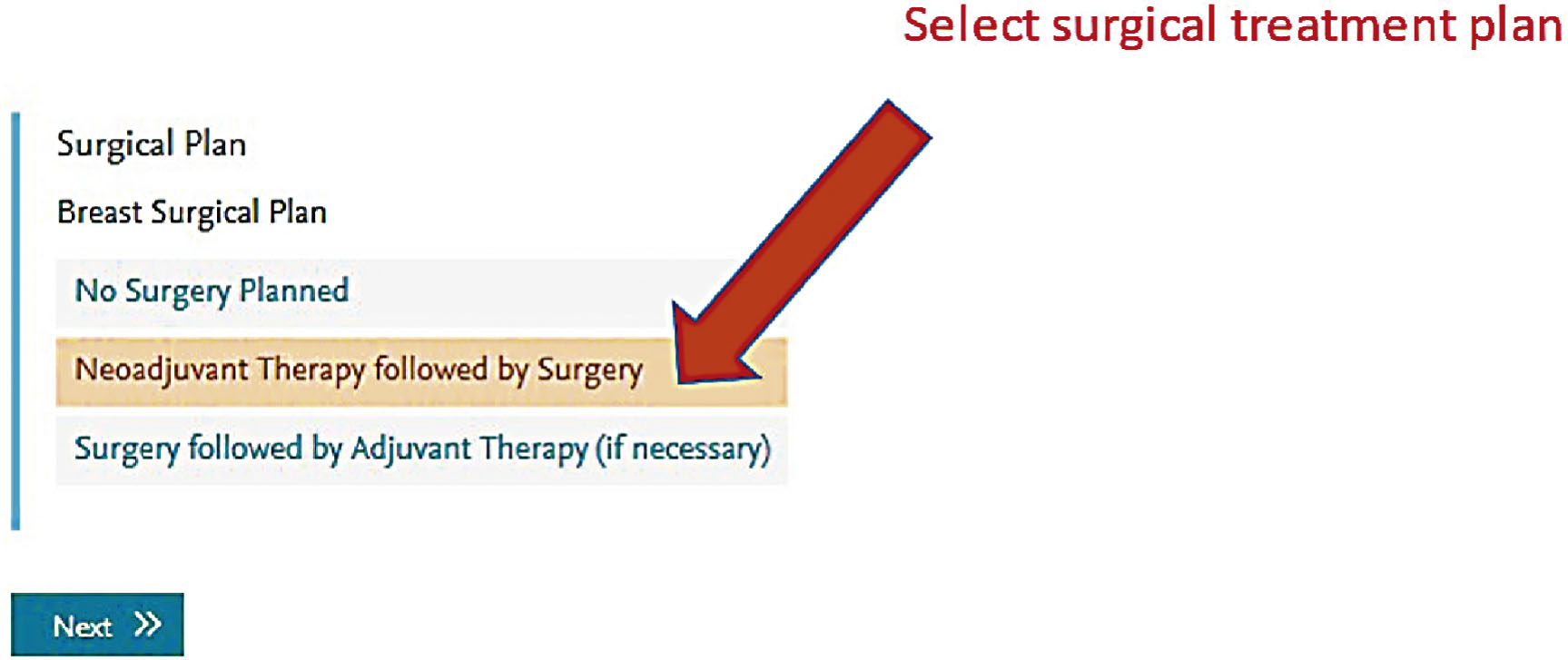

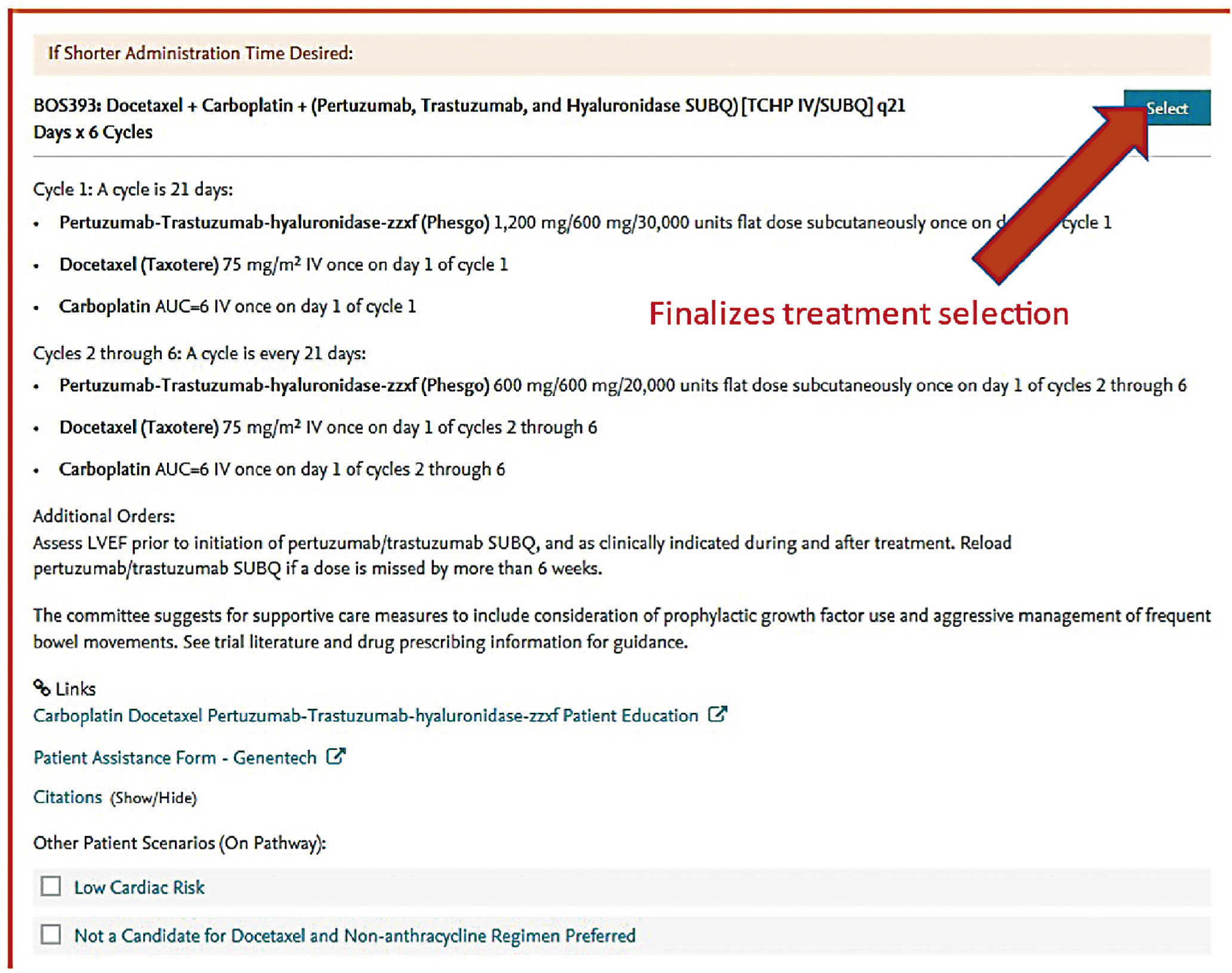
New formulations of the monoclonal antibodies targeting the HER2 domain have recently been developed and FDA approved, and are readily available in our pharmacies. These products are available for subcutaneous injection as single-agent preparations for trastuzumab, or as combination preparations for trastuzumab and pertuzumab. These formulations are considered interchangeable to the IV formulations and have quickly become part of our repertoire for patients in the neoadjuvant and adjuvant setting.
An example of the pathway that can be used in this setting is shown below:
Endocrine Therapy
The approach to neoadjuvant therapy with endocrine agents has been studied in both the premenopausal and postmenopausal setting, with significant and notable differences. Much of the comparison of the neoadjuvant approach in treatment (principally chemotherapy vs. endocrine therapy) is based on the toxicity profile and response rates achieved, and many clinical trials are designed around these two endpoints and do not give a lot of information in regard to long-term outcomes such as event-free or OS.
Several clinical trials have shown that neoadjuvant endocrine therapy is inferior in producing high rates of clinical and pathologic response in premenopausal patients. However, this is in contrast to other data that show quite comparable response rates and breast-conservation rates in postmenopausal patients receiving either neoadjuvant endocrine therapy or neoadjuvant chemotherapy, as demonstrated in a meta-analysis including almost 3500 patients. Based on this available data, one can anticipate a response rate to neoadjuvant endocrine therapy in the 60–70% range; however the complete pathologic response rate is only 1–3% after 3 months of exposure. This does not differ significantly from neoadjuvant chemotherapy outcomes for these ER-positive postmenopausal patients. Long-term follow-up in this clinical dataset shows no significant difference in local recurrences, and toxicity rates are significantly improved with endocrine therapy compared to chemotherapy, which is of course not surprising.
Based on this data, neoadjuvant endocrine therapy is typically reserved for patients who are treated in the postmenopausal setting. In the rare setting of a premenopausal patient presenting to our institutions with indication for neoadjuvant endocrine therapy, we typically strive to utilize ovarian suppression (OFS) with aromatase inhibitor (Ai)-based endocrine therapy as our treatment approach.
It is well understood that the full benefit of neoadjuvant endocrine therapy may not be achieved until at least 3–4 months of therapy, and in many instances not until even later. At our institutions, we typically administer neoadjuvant endocrine therapy in the appropriate setting for at least 4 months, and in many settings much longer. Much of the data that was accumulated more recently during the COVID-19 pandemic was driven by the fact that definitive surgical interventions had to be delayed to spare ventilator use in the operating room, and to delay patient exposure to the hospital setting. In that, we have used neoadjuvant endocrine therapy in patients that may otherwise not have qualified for neoadjuvant therapy (e.g., smaller tumors, node-negative disease) and have seen quite significant responses even in small tumors. With that, the indication for neoadjuvant endocrine therapy has broadened and is typically utilized in patients who meet criteria for endocrine therapy and do not have clinical, pathologic, or molecular evidence of benefiting from chemotherapy (Oncotype, MammaPrint), but would benefit from a neoadjuvant treatment approach based on disease extent or based on a desire to delay definitive surgical intervention. The treatment we utilize are AIs, which we use essentially interchangeably in this setting (anastrozole, letrozole, exemestane). Although there is some preliminary data on the efficacy of fulvestrant, this is not routinely used at our institutions in the neoadjuvant setting.
Immunotherapy
The addition of immunotherapy to neoadjuvant treatment has been explored in multiple settings and achieved FDA approval based on the KEYNOTE-522 trial in June 2021.
This trial showed that the addition of pembrolizumab to neoadjuvant multiagent chemotherapy containing Taxol, carboplatin, Adriamycin, and Cytoxan improves the pathologic complete response rates from 51% to 65%, regardless of the PD-L1 status. This translated to a 37% improvement in event-free survival at the subsequent updated analysis, and a measurable benefit was seen across patients with either node-positive or node-negative presentation. With this data emerging, the KEYNOTE-522 regimen has become the standard of care at our institutions, and at this time is considered for all patients fit for this treatment and considered to be good candidates for neoadjuvant therapy in the triple-negative setting.
The IMpassion031 clinical trial utilized atezolizumab in addition to nab-paclitaxel, Adriamycin, and Cytoxan in the neoadjuvant setting, and showed very promising results with respect to improvement in complete pathological response rates that increased from 41% to 58%. This clinical trial was not powered to assess long-term differences in event-free survival rates. This stands in contrast to the NeoTRIPaPDL1 trial, which showed no improvement with the addition of atezolizumab to carboplatin and nab-paclitaxel-based neoadjuvant chemotherapy. With that, atezolizumab is not currently the standard of care in the neoadjuvant triple-negative breast cancer setting at our institutions.
As much as immunotherapy has very recently changed the paradigm in the treatment of neoadjuvant therapy for triple-negative breast cancer, it has also created some additional complexities, including questions in the adjuvant setting after surgery. The KEYNOTE-522 regimen utilizes additional therapy with pembrolizumab in the adjuvant setting, and that competes with other current standards of care that include the recommendation for adjuvant therapy with Xeloda in patients having significant residual disease after neoadjuvant treatment (CREATE-X trial), and adjuvant poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitor therapy for patients carrying the BRCA mutation (Olympia trial). This means that adjuvant therapy in triple-negative disease is highly individualized after neoadjuvant therapy followed by surgery. The treatment selection is based on multiple disease and patient-specific characteristics, and is typically managed in consultation with the multidisciplinary team that includes a surgeon, radiologist, radiation oncologist, medical oncologist, geneticist, pathologist, and often a plastic surgeon.
Post-Treatment Assessment and Management
Since one major goal of neoadjuvant therapy is to aid in surgical resection, we typically aim to proceed with definitive surgical resection within 4–6 weeks after completion of neoadjuvant treatment. The same clinical and radiographic evaluation utilized pretreatment should be repeated at that time, and typically includes a contrast-enhanced breast MRI, unless the radiologist feels that the pretreatment ultrasound was more effective in assessing the true extent of the disease. The role of repeat radiographic assessment is less clear in patients who had pretreatment evidence of multicentric disease or other medical or surgical contraindications for breast conservation, in which setting pursuit of mastectomy without post-neoadjuvant treatment imaging is reasonable.
Principles of Surgery in the Neoadjuvant Setting: Management of the Primary Tumor and the Axilla
Whether the patient is ultimately a candidate for breast conservation following neoadjuvant therapy depends on the response to this treatment and the size of the residual disease in relation to the patient’s breast size. In the event of multicentric disease at presentation, breast conservation is not recommended even if a complete clinical/radiographic response is noted. Of course, a multitude of personal and other medical/surgical factors will influence the choice for mastectomy versus breast conservation. The surgical management of the primary breast tumor is discussed in the surgery chapter, and generally also applies here in the post-neoadjuvant setting.
Patients with initially clinically and radiographically node-negative disease typically are subjected to sentinel lymph node biopsy at the time of definitive breast surgery following neoadjuvant treatment.
Patients who initially present with radiographically suspicious lymph nodes undergo pathologic evaluation of those lymph nodes via core biopsy prior to neoadjuvant treatment in our institutions. The biopsied lymph nodes are marked with a radiopaque device and the marked and pathologically involved lymph node is required to be surgically removed at the time of axillary staging. Pre-neoadjuvant sentinel lymph node biopsy is generally discouraged, but in rare circumstances is indicated to clarify the pretreatment staging.
For patients who have extensive nodal involvement prior to neoadjuvant therapy (cN2 or cN3 disease), we generally still perform more generous axillary dissections at the time of definitive surgical intervention even if clinically and radiographically significant or even complete responses are encountered. However in the setting of N1 disease, the management of the axilla depends on the response to treatment. Patients who still have clinically and/or radiographically evident lymph node involvement are typically subjected to an axillary dissection, whereas patients who have clinically and radiographically excellent responses to neoadjuvant therapy are assessed with sentinel lymph node biopsy. In that setting, we require that the previously biopsied and involved lymph node (identified by radiopaque marker) needs to be part of the axillary/sentinel lymph node sampling. In order to aid in that requirement, it has become our standard practice to mark involved lymph nodes with a Savi scout that can be intraoperatively detected by the surgeon.
Principles of Radiation in the Neoadjuvant Setting
Adjuvant radiation therapy is offered to all patients undergoing breast conservation following neoadjuvant therapy, as would be considered the standard of care in any other breast-conserving setting. For patients with persistent lymph node involvement after neoadjuvant treatment, comprehensive nodal radiation is offered to all patients as well. We offer sentinel lymph node biopsy only in patients who had limited (N1) clinical/pathologic evidence of lymph node involvement prior to neoadjuvant therapy but were successfully downstaged at the time of surgery (ypN0). The role of nodal radiation therapy in this setting remains unclear at this time and multiple clinical trials are currently ongoing that are investigating the role of radiation versus observation in this particular setting. Many of our radiation oncology groups are participating in those trials.
Pathology Assessment Post-Neoadjuvant Therapy
The pathologist plays a key role in the assessment of the patient’s specimen following neoadjuvant therapy. With the advent and rapid adoption of neoadjuvant therapy in breast cancer, the American Joint Committee on Cancer updated its staging system to include a category of staging criteria specifically designed to assess disease extent following neoadjuvant therapy (ypT and ypN staging).
In addition to the anatomic staging assessment, residual cancer burden (RCB) calculators have been developed. These provide a standardized approach to the assessment of residual disease in the tumor bed and lymph nodes following neoadjuvant therapy. Scores calculated utilizing this tool are known to correlate tightly with recurrence-free survival at 10 years and are divided into RCB-0, which is synonymous with a complete pathologic response, and then range from RCB-I through RCB-III. We recently updated the requirements for our system-wide pathology reporting to include the RCB score in all patients receiving neoadjuvant therapy. Using the RCB to estimate a patient’s risk of disease recurrence helps the medical oncologist tailor additional adjuvant therapy.
Adjuvant Therapy After Neoadjuvant Treatment
For most patients undergoing neoadjuvant therapy, systemic treatment does not stop at the time of surgery.
Patients with ER-positive disease typically receive at least 5 years of adjuvant endocrine therapy regardless of their response to the neoadjuvant treatment. For premenopausal patients, especially those under the age of 35 years and who resume menstrual periods following neoadjuvant therapy, we also recommend ovarian function suppression as part of the endocrine therapy. In patients with significant lymph nodal involvement, the addition of abemaciclib has been shown to benefit patients in the adjuvant setting and is typically included for 2 years following their definitive surgery, in addition to the routine standard endocrine therapy.
Patients with triple-negative disease who still have residual disease following neoadjuvant treatment are commonly subjected to additional adjuvant therapy with either Xeloda, pembrolizumab, or a PARP inhibitor. The systemic adjuvant therapy chosen depends on several factors: if they received neoadjuvant chemoimmunotherapy following the KEYNOTE-522 regimen (pembrolizumab), if they have significant residual disease (Xeloda based on the CREATE-X trial), or if they are BRCA mutation carriers (PARP inhibitor).
In patients with HER2/neu-positive disease, the adjuvant therapy routinely includes continued HER2/neu-targeted monoclonal antibody therapy with either trastuzumab and pertuzumab or TDM1 for the total duration of 1 year. Based on the KATHERINE clinical trial, TDM1 is the preferred agent in patients who have residual disease following neoadjuvant therapy.
Adjuvant Treatment of Breast Cancer
Adjuvant therapy is an additional cancer treatment given after the primary treatment to lower the risk of cancer recurrence. The adjuvant therapy decision is based on tumor characteristics and its impact on the risk of cancer recurrence with local therapy, any prior neoadjuvant therapy, as well as the benefit and toxicity of the adjuvant therapy and the patient comorbidities.
The adjuvant systemic therapy decision in breast cancer is based on hormone receptor (HR) and HER2 status, as well as the risk of cancer recurrence based on anatomic and pathologic characteristics. In this section, we will discuss systemic adjuvant therapy including chemotherapy and endocrine therapy, as well as HER2-targeted therapy. Recently approved PARP inhibitors and cyclin-dependent kinase (CDK)4/6 inhibitors will also be discussed. Briefly, the supportive role of bisphosphonates in the adjuvant setting and the role of immunotherapy will also be discussed.
The HR-positive and HER2-negative breast cancer subtype is the most common subtype, with an age-adjusted rate of 87.4 new breast cases per 100,000 women based on 2015–2019 cases. This is followed by the HR-negative/HER2-negative subtype with an age-adjusted rate of 13.2 new cases per 100,000, HR-positive/HER2-positive with an age-adjusted rate of 12.9, and HR-negative/HER2-positive with an age-adjusted rate of 5.2.
Hormone Receptor–Positive and HER2-Negative Subtype
Most patients with HR-positive/HER2-negative breast cancer will not derive benefit from the addition of adjuvant chemotherapy to endocrine therapy. Several gene expression assays are commercially available to help select women who will benefit from the addition of chemotherapy to adjuvant endocrine therapy.
Multigene Assays
Multigene assays are approved in HR-positive and HER2-negative, node-negative as well as in one to three node-positive groups and include 21-gene (Oncotype Dx), 70-gene (MammaPrint), 50-gene (Prosigna), and EndoPredict 21-gene recurrence assays. OncotypeDx is the only assay that provides predictive information regarding chemotherapy benefit as well as prognostic information, with all other assays providing prognostic information only and their ability to predict chemotherapy benefit is unknown. The Breast Cancer Index predicts the benefit of extended adjuvant endocrine therapy.
The 21-gene recurrence score (RS) assay is based on a 21-gene (16 cancer-related genes and five reference genes) panel performed on paraffin-embedded tumor tissue and remains the most validated assay. The RSs range from 0 to 100, with higher scores indicating a greater risk of recurrence.
The OncotypeDx assay was studied in TAILORx, a prospectively conducted clinical trial that enrolled 10,273 patients between April 7, 2006 and October 6, 2010 with tumors of 1.1–5.0 cm in the greatest dimension and also included tumors measuring 0.6–1.0 cm in the greatest dimension with intermediate or high tumor grade. Patients with a RS of 0–10 were assigned to receive endocrine therapy alone, and those with a RS of 26 or higher were assigned to receive chemotherapy plus endocrine therapy. The patients with a RS of 11–25 were randomized to endocrine therapy or chemoendocrine therapy. In total, 15.9% of women had a RS of 0–10 and were treated with endocrine therapy alone. The trial confirmed that a low RS (0–10) has a low rate of distant recurrence when patients were treated with endocrine therapy alone, with a rate of 5-year invasive disease–free survival (IDFS) of 93.8% (95% confidence interval [CI], 92.4–94.9), a rate of freedom from recurrence of breast cancer at a distant site of 99.3% (95% CI, 98.7–99.6), and a rate of OS of 98.0% (95% CI, 97.1–98.6). An update in 2018 showed a low percentage of women with distant recurrence (3%) at 9 years with endocrine therapy alone if the RS was 0–15, irrespective of age.
In 2018, results in patients with a mid-range RS of 11–25 showed that endocrine therapy was noninferior to chemoendocrine therapy in the analysis of IDFS. At 9 years, the two treatment groups had similar rates of IDFS (83.3% in the endocrine therapy group and 84.3% in the chemoendocrine therapy group), freedom from disease recurrence at a distant site (94.5% and 95.0%) or at a distant or locoregional site (92.2% and 92.9%), and OS (93.9% and 93.8%). The chemotherapy benefit for invasive disease–free survival varied with the combination of RS and age ( P = .004), with some benefit of chemotherapy found in women 50 years of age or younger with a RS of 16–25 (chemotherapy benefit of 1.6% with a RS of 16–20 and 6.5% with a RS of 21–25). A secondary analysis of the TAILORx trial was performed to determine whether the patient’s age, tumor size, and histologic grade add prognostic information to the 21-gene RS and predictive information regarding the benefit of chemotherapy. This analysis showed that clinical risk stratification provided prognostic information that, when added to the 21-gene RS, could be used to identify premenopausal women who could benefit from more effective therapy.
The OncotypeDx assay was studied in HR-positive and HER2-negative breast cancer patients with one to three metastatic nodes in the RxPONDER trial. This was a large, prospective trial that randomized 5083 women (33.2% premenopausal) with a RS of 25 or less to endocrine therapy alone or to chemoendocrine therapy. The premenopausal women who received chemoendocrine therapy had longer IDFS and distant relapse-free survival (DRFS) than those who received endocrine-only therapy, whereas postmenopausal women with similar characteristics did not benefit from adjuvant chemotherapy. However, it is somewhat controversial regarding how much benefit there was for premenopausal women in the chemoendocrine arm because of OFS caused by chemotherapy rather than the direct cytotoxic effect of the chemotherapy alone. Also, in clinical practice, this data is to be used with caution in patients with the high-grade disease with three positive nodes, as only 10.3% of patients had high-grade disease and 9.2% had three involved nodes.
The 70-gene assay (MammaPrint) has also been studied in a prospective phase III randomized trial, MINDACT. This trial, which enrolled 6693 early breast cancer (N0 or one to three nodes positive) patients demonstrated that the 70-gene assay (MammaPrint) can identify a subset of patients who have a low likelihood of distant recurrence despite high-risk features based on size, grade, and nodal status (1.5% chemotherapy benefit in 5-year survival when added to endocrine therapy). In this trial, women at low clinical and genomic risk did not receive chemotherapy, whereas those at high clinical and genomic risk did receive such therapy. In patients with discordant risk results, either the genomic risk or the clinical risk was used to determine the use of chemotherapy. With a more mature follow-up approaching 9 years, the 70-gene signature shows an intact ability to identify among women with high clinical risk a subgroup with low genomic risk, with an excellent distant metastasis–free survival when treated with endocrine therapy alone. For these women, the magnitude of the benefit from adding chemotherapy to endocrine therapy remains small (2.6%) and is not enhanced by nodal positivity. However, in an underpowered exploratory analysis, this benefit appears to be age dependent, as it is only seen in women younger than 50 years, where it reaches a clinically relevant threshold of 5%. However like the 21-gene RS assay, the concern is that the noted benefit could be due to chemotherapy-induced ovarian function suppression rather than the direct effect of chemotherapy. This needs to be part of informed, shared decision-making with the patient.
Adjuvant Endocrine Therapy
Patients with invasive breast cancer that is ER- or PR-positive should be considered for adjuvant endocrine therapy regardless of the patient’s age, lymph node status, or whether adjuvant chemotherapy was given. The endocrine options for women depend on whether they are in menopause or not. Menopausal status cannot be determined in patients receiving ovarian function suppression. There are no evidence-based criteria for the diagnosis of menopause. The National Comprehensive Cancer Network defines menopause as:
- •
Women 60 years and older
- •
Women less than 60 years are postmenopausal if one of the following conditions is met:
- •
They previously underwent a bilateral oophorectomy.
- •
Amenorrhea for 12 months or more in absence of tamoxifen, toremifene, prior chemotherapy, or OFS, and serum estradiol and follicle-stimulating hormone (FSH) is in the postmenopausal range.
- •
They are amenorrheic on tamoxifen and serum estradiol and FSH are in the postmenopausal range.
- •
Chemotherapy-induced amenorrhea for 12 months or more with estradiol and FSH levels in the postmenopausal range on serial assessments.
- •
Only tamoxifen and AIs are approved medications for adjuvant treatment of breast cancer.
Tamoxifen is a selective estrogen receptor modulator (SERM) and can be used in ER-positive premenopausal and postmenopausal women. SERMs are competitive inhibitors of estrogen binding to the ER and have mixed agonist and antagonist activity. Tamoxifen has a prominent antagonist effect in breast cancer and is the only SERM approved to be used in the adjuvant setting.
Tamoxifen is converted to its active metabolites by CYP2D6 and UGT2B7. Some selective serotonin reuptake inhibitors such as fluoxetine and paroxetine inhibit CYP2D6 and decrease the formation of endoxifen, 4OH-tamoxifen, and active metabolites of tamoxifen, which may impact the efficacy of tamoxifen. Serotonin and norepinephrine reuptake inhibitors appear to have minimal impact on tamoxifen. These drug interactions should be considered when prescribing these medications but are not a contraindication. Studies have failed to demonstrate a survival difference with the use of selective serotonin reuptake inhibitors among patients taking tamoxifen.
Side effects of tamoxifen include hot flashes, sexual dysfunction, venous thromboembolism (two- to threefold increase in relative risk), abnormal uterine bleeding, as well as increased risk of endometrial cancer and uterine sarcoma and a slight increase in the risk of stroke. Tamoxifen is also associated with fatty liver disease in over one-third of patients despite a favorable effect on cholesterol levels. There has been an increased risk of cataracts and less commonly reversible corneal pigmentation and irreversible retinal deposits that can be associated with macular edema and vision loss. Premenopausal women can remain fertile during tamoxifen treatment and should be strongly advised to use effective contraception while on tamoxifen treatment. Tamoxifen is related to birth defects and women planning to get pregnant should stop tamoxifen 2 months prior to attempting pregnancy.
Duration of Treatment
A total of 5 years of tamoxifen decreases the annual odds of recurrence by 39% and annual odds of death by 31% irrespective of use of chemotherapy, age, lymph node status, and menopausal status, and was significantly more effective than 1–2 years’ use.
A meta-analysis of the results of 88 trials involving 62,923 women with ER-positive breast cancer who were disease free after 5 years of scheduled endocrine therapy showed that the breast cancer recurrences continued to occur steadily throughout the study period from 5 to 20 years. The risk of distant recurrence was strongly correlated with the original tumor and nodal status, with risks ranging from 10% to 41%, depending on tumor and nodal status and tumor grade.
In trials of 5 years of tamoxifen therapy versus no endocrine therapy, the recurrence rate in the tamoxifen group was approximately 50% lower than that in the control group during the first 5 years (the treatment period) and approximately 30% lower during the next 5 years. The rate of death from breast cancer in the tamoxifen group was approximately 30% lower than that in the control group during the first 15 years (i.e., including 10 years after the cessation of therapy).
Clinical trials comparing 5 or 10 years of tamoxifen include ATLAS and the aTTom trials. The ATLAS trial randomized women to 5 or 10 years of adjuvant tamoxifen, and the risk of recurrence during years 5–14 improved from 25.1% to 21.4% with 10 years of tamoxifen, with an absolute mortality reduction of 2.8%. There was an increased risk of endometrial cancer and pulmonary embolism. The aTTom trial confirmed the significant reduction in recurrence with continuing tamoxifen to year 10. Breast cancer mortality was reduced by about one-third in the first 10 years following diagnosis and by half subsequently.
Tamoxifen, when used with chemotherapy, should always be used sequentially with chemotherapy given first followed by tamoxifen, based on SWOG 8814 data.
Role of Ovarian Suppression in Premenopausal Women
Before the availability of tamoxifen, OFS as a single treatment modality was utilized with some benefit. In contemporary medicine OFS alone is not considered standard-of-care adjuvant systemic therapy for breast cancer. For women with early-stage HR-positive breast cancer, adjuvant treatment with tamoxifen reduces their 15-year risk of death from breast cancer by about one-third. AIs are even more effective than tamoxifen in postmenopausal women but, used alone, are ineffective in premenopausal women due to compensatory ovarian estrogen production. Several trials have assessed whether, if administered with OFS, AIs may also be more effective than tamoxifen at preventing breast cancer recurrence in premenopausal women, but trial results have been inconsistent.
OFS has been used for multiple years in premenopausal women with controversial data. The 8-year update of the SOFT and TEXT trials compared 5 years of tamoxifen alone, tamoxifen with OFS, or exemestane with OFS. SOFT showed that the addition of OFS resulted in significantly higher (83.2% with OFS plus tamoxifen and 85.9% with OFS plus exemestane) 8-year disease-free survival (DFS) compared with tamoxifen alone (78.9%). Among the women with cancers that were negative for HER2 who received chemotherapy, the 8-year rate of distant recurrence with exemestane plus OFS was lower than the rate with tamoxifen plus OFS (by 7% in SOFT and by 5% in TEXT). There were higher grade 3 toxicities with OFS.
13 year median follow up update of the combinaed analysis of SOFT-TEXT trials in intention-to-treat population showed 12 year absolute improvement of 4.6% in disease free survival with 1.8% absolute improvement in distant recurrence free interval but no overall survival improvement in patients assigned to exemestane+OS over tamoxifen+OS. Among patients with HER2 negative tumors (86% of the intention-to-treat population) the absolute improvement in 12 year overall survival with examestane +OS was 2% and it was 3.3% in those who recieved chemotherapy (45.9% of the intention-to-treat population). Overall survival benefit was clinically significant in high-risk patients, eg, women age < 35 years (4.0%) and those with > 2 cm (4.5%) or grade 3 tumors (5.5%).
A meta-analysis of 7030 women enrolled in four randomized trials evaluated AIs versus tamoxifen with OFS in premenopausal women. The 5-year absolute risk of breast cancer recurrence was 3.2% lower in the AI group than the tamoxifen group (6.9% vs. 10.1%) with no difference in breast cancer mortality.
Aromatase Inhibitors
AIs suppress plasma estrogen levels by inhibiting or inactivating the enzyme aromatase, which is responsible for peripheral conversion of androgens to estrogens. AIs are only active in women with nonfunctioning ovaries.
There are three AIs available: anastrozole, letrozole, and exemestane. All three have shown similar antitumor efficacy and toxicity profiles in randomized trials. AIs are associated with hot flashes, night sweats, loss of bone density, musculoskeletal pains and stiffness, and sexual dysfunction. AI-associated musculoskeletal syndrome includes joint stiffness, arthralgias, bone pain, and carpal tunnel syndrome. These symptoms may be severe in up to one-third of the patients and lead to discontinuation of treatment in 10–20% of patients. There is data that exercise, nonsteroidal anti-inflammatory drugs, duloxetine, acupuncture, and brief interruption of the AI treatment may help.
If AIs are not tolerated, then a change to tamoxifen to complete the required adjuvant endocrine therapy is recommended.
Aromatase Inhibitors and Tamoxifen
The ATAC trial demonstrated anastrozole to be superior to tamoxifen with improved DFS (2.7% at 5 years and 4.3% at 10 years) with no difference in OS.
Several studies have evaluated AIs as initial adjuvant therapy or sequential with tamoxifen. The BIG 1-98 study compared 5 years of tamoxifen alone, letrozole alone, or sequential use of the drugs and showed a 9% relative reduction in the hazard of a DFS event with letrozole compared with tamoxifen. Outcomes achieved with the sequence of letrozole taken for 2 years followed by tamoxifen for 3 years were close to those with 5 years of letrozole monotherapy. This data is similar to the TEAM trial, where 5 years of the sequential regimen was similar to the AI-alone arm.
Several trials have studied the use of tamoxifen for 2–3 years followed by AIs versus continued tamoxifen. A meta-analysis of the ABCSG-8, ARNO 95, and ITA studies showed significant improvement in DFS and OS with a switch to AIs.
Duration of Endocrine Therapy
Similar to tamoxifen, there is evidence for benefit of extended adjuvant endocrine therapy in patients initially treated with tamoxifen. MA17 compared 5 years of tamoxifen compared with 5 years of tamoxifen followed by letrozole. The letrozole group showed improvement in DFS, but OS improvement was noted only in the lymph node-positive patients. MA17-R evaluated extending AIs beyond 5 years. There was an improvement in 5-year DFS in patients on letrozole compared with placebo (95% vs. 91%), with no benefit in OS. However, there was an increase in bone fracture (14% vs. 9%), new-onset osteoporosis (11% vs. 6%), and bone-related events (18% vs 14%) with a longer duration of AIs. A 10-year analysis of NSABP B-42 was presented at San Antonio Breast Cancer Symposium 2019 and also showed statistically significant improvement in DFS with an absolute improvement of 4% but no improvement in OS with the use of extended letrozole after 5 years of hormonal therapy.
ABCSG-16/SALSA is a prospective, phase III trial that compared 2 versus 5 additional years of AIs in postmenopausal HR-positive women after 5 years of endocrine therapy. After 10 years of follow-up, no benefit was noted for 5 additional years compared with 2 additional years of treatment with respect to DFS or OFS, or the risk of contralateral breast cancers. However, the majority of patients on this trial had low-risk T1 and N0 breast cancers that had been treated without adjuvant chemotherapy. Most patients had received tamoxifen alone (51%) or tamoxifen sequenced with an AI (42%) before trial entry.
Role of Cyclin-Dependent Kinase 4 and 6 Inhibitors
Patients with breast cancer who have multinodal involvement, especially with four or more ALNs, have at least a 38% risk of relapse from 5 to 20 years. Patients with one to three ALNs and another poor prognostic factor are linked with a greater than 23% risk of relapse. Despite successes observed with endocrine therapy for this patient population, there is an unmet need for identifying those who have primary endocrine resistance and preventing or delaying their recurrence with additional treatment.
CDK4/6 are important regulators of cell cycle progression in many cell types, including ER-positive breast cancer. In randomized trials, adding a CDK4/6 inhibitor to an AI or fulvestrant improves PFS and OS in both premenopausal and postmenopausal women. Multiple clinical trials have evaluated the role of CDK4/6 inhibitors in high-risk early breast cancer.
Palbociclib was studied in a prospective, randomized, phase III PALLAS trial. A total of 5796 patients with HR-positive and HER2-negative early breast cancer were randomly assigned to receive 2 years of palbociclib (125 mg orally once daily, days 1–21 of a 28-day cycle) with adjuvant endocrine therapy or adjuvant endocrine therapy alone (for at least 5 years). There was no improvement in IDFS or any other efficacy end point (4-year IDFS 84.2% vs. 84.5%; hazard ratio 0.96).
PENELOPE-B is another double-blind, placebo-controlled, phase III study in which women with HR-positive HER2 – without a pathological complete response after taxane-containing NACT and at high risk of relapse were randomly assigned (1:1) to receive 13 cycles of palbociclib 125 mg once daily or placebo on days 1–21 in a 28-day cycle in addition to endocrine therapy. Palbociclib for 1 year in addition to endocrine therapy did not improve IDFS in women with residual invasive disease after NACT.
MonarchE is the only positive trial showing the benefit of adding CDK4/6 inhibitors to high-risk breast cancer with adjuvant endocrine therapy. This is a phase III trial that randomized 5637 adult women and men with HR-positive, HER2-negative, node-positive, resected, early high-risk breast cancer. Patients were randomized to receive either 2 years of abemaciclib plus their physician’s choice of standard endocrine therapy for 5 years or greater (maximum 10 years) or standard endocrine therapy alone. The trial enrolled patients with four or more positive ALNs, or one to three positive nodes and either grade 3 disease or tumor 5 cm or larger in size, or one to three positive nodes and a centrally tested Ki-67 of 20% or greater.
At the primary outcome analysis, with 19 months median follow-up time, abemaciclib plus endocrine therapy resulted in a 29% reduction in the risk of developing an IDFS event (hazard ratio, 0.71, 95% CI, 0.58–0.87; nominal P = 0.0009]. At the additional follow-up analysis, with 27 months median follow-up and 90% of patients off treatment, IDFS (hazard ratio, 0.70; 95% CI, 0.59–0.82; nominal P < .0001) and DRFS (hazard ratio, 0.69; 95% CI, 0.57–0.83; nominal P < .0001) benefit was maintained. The absolute improvements in 3-year IDFS and DRFS rates were 5.4% and 4.2%, respectively. Whereas the Ki-67 index was prognostic, abemaciclib benefit was consistent regardless of Ki-67 index. Safety data were consistent with the known abemaciclib risk profile. OS data were not mature at the time of the IDFS analysis. The most common adverse reactions (≥20%) were diarrhea, infections, neutropenia, fatigue, leukopenia, nausea, anemia, and headache. The risk of VTE was higher when used in combination with tamoxifen, with about 4% of patients in monarchE that received tamoxifen and abemaciclib experiencing a VTE.
On October 12, 2021, the FDA approved abemaciclib with endocrine therapy (tamoxifen or an AI) for adjuvant treatment of adult patients with HR-positive, HER2-negative, node-positive early breast cancer at high risk of recurrence and a Ki-67 score ≥20%, as determined by an FDA-approved test.
Although exploratory analyses suggested similar hazard ratios in favor of abemaciclib regardless of Ki-67 status, there were relatively few Ki-67–low tumors in monarchE.