Chapter Outline
Approach to the Child with Pain
Analgesic Medications and Interventions
Invasive Approaches to Pain Management
Psychological and Nonpharmacologic Approaches to Pain
Pediatric Cancer Pain Syndromes
Reassessing Interventions for Pain
Chemotherapy-Induced Nausea and Vomiting
Radiation-Induced Nausea and Vomiting
Nonpharmacologic Interventions
Role of the Patient and Family
OTHER GASTROINTESTINAL SYMPTOMS
MOOD DISORDERS AND MENTAL STATUS CHANGES
Progress in understanding the causes of pediatric cancer, as well as advances in cancer-directed therapies, hold great promise for curing and extending the lives of many children diagnosed with cancer. However, as advances in medicine and technology improve the survival of children with life-threatening illnesses, attention to health-related quality of life and progress in symptom management have not kept pace with advances in disease-directed therapies. As a result a population of children exists who are living with cancer and have suboptimally controlled symptoms.
Amelioration of symptoms experienced by a child with cancer does far more than reduce the suffering caused by the troublesome symptoms. Control of physical symptoms may allow attention to be focused on other issues faced by family members who are living with a child’s cancer, such as psychosocial concerns or existential distress, and it may allow them to participate in activities and interactions that are important for maximizing quality of life. In fact, symptom burden is highly associated with quality of life in children with cancer and is one of the most significant determinants of quality of life in adolescents and young adults with cancer. In some instances, improved control of symptoms may enhance the delivery of optimal cancer-directed therapy as well. Finally, optimal symptom control throughout the illness trajectory may also shape the child’s and family’s long-lasting impressions of their experience with cancer.
In many instances, relief from distressing symptoms is possible with a myriad of modalities available today. Pediatric oncologists play a key role in managing symptoms in their patients. Symptom management requires actively partnering with families to assess not only the presence of symptoms but also the impact of uncontrolled symptoms on their daily lives, as well as to implement appropriate symptom-directed interventions. Ideally such attention to symptoms occurs in the context of attention to the child’s overall condition so that the impact of symptoms on the child’s overall quality of life is appreciated.
The vast majority of children who have cancer experience multiple symptoms that could be ameliorated but are not addressed. For example, Collins and colleagues asked 10- to 18-year-old children about the symptoms they experienced during the past week. Symptoms included lack of energy (49%), pain (49%), nausea (45%), lack of appetite (40%), and itching (33%). The prevalence of uncontrolled symptoms in children with cancer is likely to stem from a variety of systemwide causes, including inadequate formal training dedicated to symptom management, a focus on cancer-directed treatment, and time constraints.
In addition, a lack of systematic research in the pediatric population, particularly with regard to non–pain-related symptoms, leads to a lack of evidence or standards on which to base interventions. Many symptom-directed interventions for children at this time are based on extrapolation from adult studies or even individual or anecdotal experiences. Some guidelines exist that are strongly evidence based, such as the National Comprehensive Care Network (NCCN) Guidelines for Pediatric Cancer Pain, which is encouraging. As advances in pediatric oncology extend into the realm of symptom management, additional guidelines for non–pain-related symptoms will become available to help pediatric oncologists attend to their patients’ symptoms.
The promising survival rates that have resulted from discoveries in pediatric oncology have led to increasing attention to health-related quality of life and the human costs of cancer care. With such a shift, efforts to understand and ameliorate the myriad of symptoms experienced by children with cancer are likely to be increasingly supported in the coming years.
Pain
Pain is defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.” This symptom is the most studied and best understood of all cancer symptoms in adults and children alike. However, despite such attention, pain is often suboptimally controlled.
Families and children confronting cancer often worry about potential pain due to the disease and its treatment. In fact, pain is the most feared problem for children with cancer. To some extent, their concern is justified. For example, Collins and colleagues demonstrated that children with cancer experience multiple symptoms, including pain. Among 160 children ages 10 to 18 years with cancer, pain was the second most common symptom, with a prevalence of 49.1%. In addition, 61.6% reported pain that was moderate to very severe in the 48 hours prior to completing the questionnaire, and 40.9% reported pain that occurred “a lot” to “almost always.” Moreover, the pain experienced by children was distressing—“quite a bit” to “very much” in 39.1% of respondents.
Pain control provides a variety of benefits beyond the amelioration of suffering. Prompt pain relief is needed to prevent central sensitization, a centrally mediated hyperexcitability response that may result in escalating pain. Uncontrolled pain also leads to a physiologic stress response with a variety of effects such as altered metabolism and immune function. Control of pain with appropriate analgesia in the perioperative period can reduce some of these effects and prevent complications.
Epidemiology
In general, pain experienced by children with cancer may be caused by a variety of entities, including the disease itself (e.g., tumor invasion of bone, viscera, or the peripheral or central nervous system [CNS] or compression of the spinal cord), treatment (e.g., mucositis, radiation-induced dermatitis, and drug-induced neuropathy) or procedures (e.g., venipuncture, lumbar puncture, and bone marrow aspiration or biopsy, as well as postoperative pain). Authors who performed a cross-sectional analysis of pain in inpatient children with cancer found that the most frequent cause of pain was adverse effects of antitumor therapy. Evidence also indicates that children who have solid tumors outside of the CNS have more pain and higher opioid requirements.
Because the majority of pediatric cancers respond at least initially to treatment, most of the pain that children experience early in the disease trajectory is related to procedures and treatment. Later, if the cancer progresses, pain is more likely to be due to tumor extension. In a series of structured interview surveys conducted by Ljungman and colleagues, 49% of children with cancer experienced pain at diagnosis. Procedure- and treatment-related pain were the most significant types of pain at the start, and although procedure-related pain improved, treatment-related pain did not improve. In addition, measurement of pain intensity was rarely performed.
A significant barrier to pain management in children stems from the fact that research and development of evidence-based practice guidelines in pediatrics lag behind that in adults. The NCCN and World Health Organization (WHO) have developed comprehensive guidelines for managing pain in children with cancer, but in many instances, pain management in children relies on extrapolation from adult data, anecdotal reports, and personal experience.
Children may also experience incidental, non–cancer-related pain. Other pain-inducing disorders such as migraines, recurrent abdominal pain, or injuries seen in the general pediatric population can also occur in children with cancer. These causes of pain unrelated to the cancer diagnosis should always be included in the differential diagnosis of pain in children who have cancer.
Pathophysiology
Nociception is a complex process whereby actual (or potential) tissue damage is perceived as pain by an individual. In many instances pain is a protective mechanism that alerts a person to tissue injury. Nociception may be thought to occur on three levels: peripheral, spinal, and supraspinal ( Fig. 71-1 ). Through transduction, the primary afferent nociceptors, which are thinly myelinated A-delta and unmyelinated C fibers, transmit biochemical changes at the sensory nerve endings that are generated by painful (chemical, thermal, or mechanical) stimuli into electrical signals. Stimulation of such nerve endings in the periphery may be increased or decreased by molecules such as prostaglandins, leukotrienes, bradykinin, and histamine. For example, the bradykinin and histamine that accompany tissue inflammation can directly activate nociceptors, in addition to reducing their threshold and increasing their response to suprathreshold stimulation. The analgesic effect of nonsteroidal antiinflammatory drugs (NSAIDs) lies in their ability to inhibit prostaglandin synthesis and thus desensitize nociceptors.
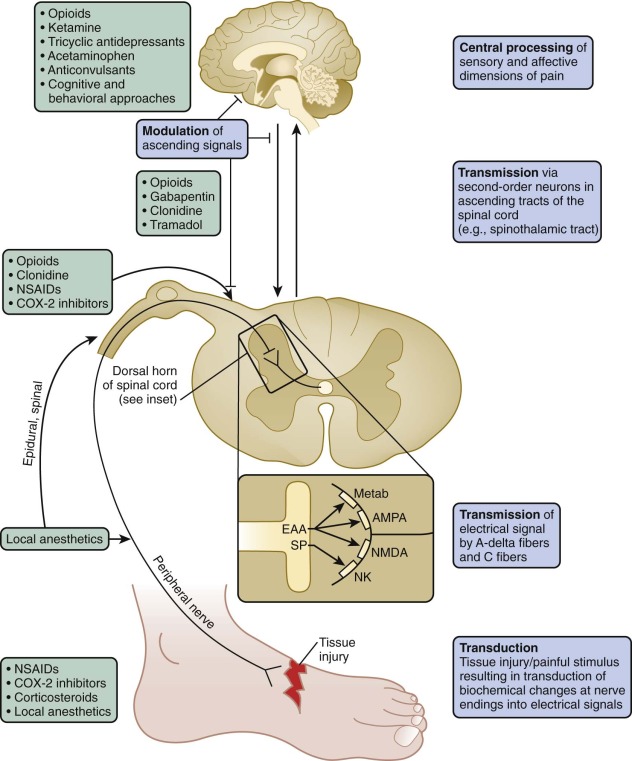
The electrical signals generated are propagated, or transmitted, to second-order neurons that synapse with sensory nociceptors in the dorsal horn of the spinal cord. Second-order neurons convey signals via ascending tracts in the spinal cord, including the anterolateral spinothalamic tracts, to supraspinal sites that include the brainstem, thalamus, and cortical areas involved in the sensory and affective dimensions of pain.
For example, descending inhibitory pathways from the thalamus and brainstem modulate excitatory transmission through such inhibitory neurotransmitters as serotonin, norepinephrine, and endogenous opioids. Several pharmacologic interventions such as opioids and tricyclic antidepressants (TCAs) exert their analgesic effects through such inhibitory processes at the spinal and supraspinal levels.
Pain is a complex sensory and emotional experience that involves nociception but is modified by a range of contextual and psychological factors. Because the experience of pain is subjective, the degree of tissue injury and therefore nociceptive input does not necessarily correlate with the intensity of pain experienced. Interventions that may reduce the perception of pain include hypnosis and relaxation techniques.
Pain that results from stimulation of intact neurons by impulses reflecting tissue injury or inflammation is called nociceptive pain . On the other hand, pain resulting from abnormal excitability of neurons (for example, due to neuronal damage) is called neuropathic pain . Even if tissue damage initially accompanied neuropathic pain, neuropathic pain may persist long after the damage has resolved. Nociceptive pain often may be distinguished from neuropathic pain by its characteristics. Neuropathic pain frequently is described as burning or shooting and is often associated with paresthesias or allodynia (i.e., elicitation of pain by normally nonpainful stimuli such as light touch).
Factors Influencing a Child’s Experience of Pain
As a subjective experience, many factors have an impact on a child’s experience of pain. Recognizing such factors can facilitate an understanding of the pain as it is experienced by the child and facilitate development of an effective pain treatment plan. The experience of pain shapes learning in infancy and throughout life. The neurobiologic mechanisms underlying nociception develop during the third trimester. Specific cortical responses to noxious stimuli can be demonstrated in 32-week preterm infants using evoked potentials or near-infrared spectroscopy. The nature of pain as a conscious experience in young infants remains a subject of speculation and controversy.
Factors influencing the perception and meaning of pain that occurs after infancy are both individual and contextual and include developmental and cognitive factors (e.g., understanding, control, expectation, and relevance) and behavioral and emotional factors (e.g., anxiety, fear, frustration, anger, guilt, and isolation), as well as familiar and cultural factors. A variety of other factors including age, gender, pain acceptance, and pain tolerance have been hypothesized to influence pain perception in the pediatric population and have been summarized elsewhere.
Importantly, previous encounters with pain may heighten the experience of subsequent encounters with pain. For example, children newly diagnosed with cancer who had inadequate procedural analgesia when undergoing a first bone marrow aspirate or lumbar puncture had more severe distress during subsequent procedures, even when efficacious pain relief was subsequently provided. This finding highlights the need to provide effective analgesia to prevent both present and future painful experiences.
Assessment
Pain assessment and measurement provide the foundation for addressing pain effectively. Regular assessment of pain may improve pain management and should be conducted in a developmentally appropriate manner. When permitted by the child’s developmental status, self-report is considered the gold standard in pain assessment. Because self-report can include bias and error, behavioral observations, physiologic changes, and clinician/parental report may be incorporated into the pain assessment. However, these methods also have inherent limitations. For example, tachycardia may reflect fever or intravascular volume depletion rather than pain.
Physiologic and behavioral signs and symptoms can indicate pain, but lack of these signs does not indicate absence of pain, particularly in chronically or very ill children, in whom these indicators are unreliable. In one study comparing a behavioral pain measure with two self-report measures, many children who reported severe pain showed few behavioral pain indicators. In addition, these signs are not necessarily specific for pain itself and may reflect distress that may or may not be related to pain. Parental and clinician estimates of the child’s pain also have limitations, because clinicians and parents frequently underestimate pain.
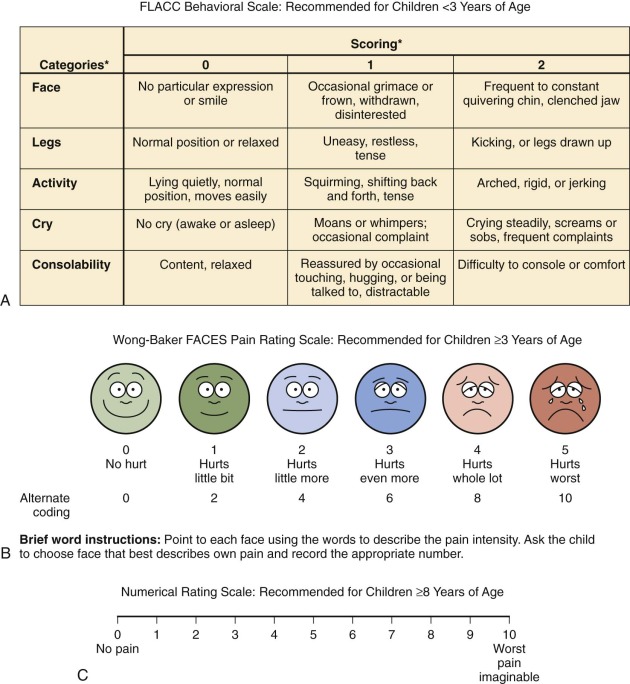
Examples of symptom assessment tools for children of various ages and developmental capacities are demonstrated in Figure 71-2 . These tools may be helpful in assessing symptoms and measuring severity before and after interventions. However, they are not all validated specifically for the population of children with cancer. A given tool should be used consistently with a given child. When the assessment reveals the presence of pain, further inquiry regarding the nature of the pain (e.g., the character of the pain and aggravating and alleviating factors) and the meaning that the pain holds for the child and the family is in order.
Self-Report Instruments
Many children 3 years of age or older are able self-report their symptoms. Because some young children, particularly those who are chronically ill, may be more mature than their chronologic age, the responses even of young children should be heeded. Self-report scales for children include faces scales, color analogue scales, visual analogue scales, the Poker Chip Tool, and numeric rating scales. With the faces scales, such as the Wong-Baker Faces Scale and the Bieri Faces Pain Scale, the child is asked to match how he or she feels with one of the faces. Color analogue scales and faces scales can be used by most children ages 4 years and older. Faces scales differ psychometrically; an example is the use of a smiling face for the “no pain” anchor in the Wong-Baker Faces Scale versus a neutral face for the “no pain anchor” in the Bieri Faces Pain Scale. Although children often report that they like using the Wong-Baker Scale, some researchers regard the use of a neutral face for the “no pain anchor,” as in the Bieri Faces Pain Scale, to be psychometrically more specific. With the visual analogue scale, a child selects a point on a line that represents the intensity of his or her pain. These scales have been extensively studied and are appropriate for children 8 years and older.
Numeric rating scales do not require any equipment, are simple to use, and are already used frequently with adults. These scales require numeracy and the ability to think and express oneself in quantitative terms and thus are most appropriate for use in children who are at least 8 years of age. Children younger than 8 years may provide an unreliable numeric response because, although they can count, they have do not have an understanding of the quantitative meaning of numbers. All quantitative scales are based on the concept of counting, which is a universal concept for children who have this developmental capacity. It is therefore possible to develop quantitative tools for pain measurement that are appropriate for children of virtually all cultures.
Behavioral Observation Scales
Behavioral and physiologic cues are particularly useful in preverbal children and in children who are not able to verbalize their symptoms because of cognitive impairment, developmental capacity, or sedation. Such scales may rely on facial expressions, motor or verbal responses, or combinations of behavioral and autonomic responses. Behavioral scales may actually rate distress, including fear and anxiety, rather than specifically assessing pain per se.
When using behavior to assess pain, it is important to partner with parents or other caretakers who are particularly familiar with the child, because knowing the child, having familiarity with children who have the same or similar conditions, and having a thorough grasp of the science of symptom management are all important components of effective pain relief. For example, the Paediatric Pain Profile was created to be a usable document for parents to assess and record their child’s pain behavior. It is a well-validated instrument that uses behavioral cues, including changes in facial expression, vocal sounds, posture and movements, sleeping, eating, and mood, to assess pain. The Individualized Numeric Rating Scale is an individualized scale for nonverbal children. This scale is an adaptation of the numeric rating scale; it allows parents or other clinicians who know the child well to identify the child’s typical pain behavior and rank that behavior on a standard scale from 0 to 10.
The Face, Legs, Activity, Cry, Consolability (FLACC) tool was originally designed to score postoperative pain in children ages 2 to 7 years but has also been validated for postoperative use in children with cognitive impairment. Use of this instrument may be advantageous because it employs a variety of types of measures such as activity and facial expression. Further work in the area of pain assessment in children with developmental or cognitive impairment is needed because children in these vulnerable populations are less likely to be assessed for pain, in addition to receiving less analgesic medication.
The previously discussed assessment scales largely reduce pain assessment to the measurement of pain intensity. Although such pain assessment is an oversimplification, the scales permit rapid evaluation of pain and rapid institution of interventions aimed at ameliorating pain. Such scales are also key outcome measures in evaluations of pain-relieving interventions. Findings from a recent study, however, highlight the fact that such a simple screening for pain intensity fails to identify many persons who have significant functional interference from pain or pain significant enough to trigger a physician visit. Regardless of the scale used, if clinical pain indicators are unclear, a trial of measures to ameliorate pain may clarify the cause of distress or pain.
Beyond Assessment Instruments
Beyond use of formal assessment tools, key elements in the history include alleviating/exacerbating factors; the quality, location, onset, and severity of pain; and the degree of impact on the child’s function and well-being. Understanding the child’s previous experiences with pain and strategies that have been used successfully in the past to address pain are also key components of the history and may inform the treatment plan. To this end a multidimensional indicator of pain can help the clinician understand the existence, intensity, and location of pain that a child is experiencing. Use of techniques that employ more than one measure (e.g., self-report, behavioral, and physiologic) permit a more accurate pain assessment.
In addition, it is necessary to understand the holistic nature of pain, rather than pain as merely a physiologic phenomenon. For example, the meaning of the pain, the degree of distress it causes, and the impact of pain and pain treatment on the child’s functional capacity and quality of life are crucial elements of the history. Exploration of these issues may elicit exacerbating and potentially modifiable factors related to the pain experience. Finally, it is important to gain a clear understanding of the beliefs that the child and family have about pain, as well as their treatment goals in terms of pain control.
Approach to the Child with Pain
Anticipation and prevention of pain is the most effective treatment approach. Just as children and parents need information regarding their cancer treatment so they know what to expect, informing them in a sensitive manner about the symptoms they may expect and strategies available for reducing their symptoms can reduce anxiety about the unknown. In many cases, without open conversations in which expectations can be addressed, the imagined reality that is substituted is far worse than the true reality. By helping children understand what will happen and how the treatment or procedure will work, their fear and anxiety may be allayed, thereby reducing the symptoms they may actually experience.
When a child reports pain, careful assessment with use of history and physical examination is imperative to generate a complete differential diagnosis. Determination of the underlying cause of pain may inform consideration of which modalities are likely to be most effective in alleviating it. Both pharmacologic and nonpharmacologic (e.g., cognitive, behavioral, physical, and supportive) approaches should be considered, including addressing factors that are likely to affect the child’s experience of pain. Once a pain treatment plan is implemented, pain should be regularly reassessed. The goal of pain management should be to achieve the degree of comfort that the child finds satisfactory.
To provide optimal pain control and prevent breakthrough pain, analgesics should in most cases be administered “By the ladder, by the clock, by the appropriate route, by the child” (WHO), and behavioral, physical, and cognitive supports should be provided throughout treatment. Pain of a moderate to severe rating should be treated with analgesics that are administered around the clock, with rescue doses of the same or an alternative analgesic available. Families often find it helpful to understand that preemptive analgesia is far more effective than catch-up analgesia administered for established pain.
Finally, the dosing interval as determined by the pharmacokinetics of the agents considered and the feasibility of their administration by caregivers should also be considered.
WHO Guidelines
The WHO guidelines previously provided a three-step approach to cancer-related pain in children and adults. For mild pain in a child who is not taking any analgesics, an NSAID or acetaminophen was suggested. For moderate pain, a short-acting weak opioid (e.g., codeine) was recommended, and for severe pain, a strong opioid was recommended (e.g., morphine).
The three-step WHO guidelines, which were widely publicized for cancer-related pain, provided an effective approach to pain control for many adults, with published success rates of 69% to 100%. However, the three-step ladder consisting of nonopioids, weak opioids, and strong opioids may not be appropriate for some patients with cancer, particularly those with advanced disease. In fact, studies have shown that a two-step ladder in which treatment passes directly from step I (nonopioids) to step III (strong opioids) for mild to moderate pain provides superior pain relief. Some medications historically used in step II (e.g., codeine and tramadol) are unlikely to provide better control of moderate pain compared with continuation of acetaminophen and NSAIDs (unless contraindicated) along with titrated doses of a strong opioid. Furthermore, codeine presents safety and efficacy concerns (discussed later), and few data exist to guide the administration of tramadol or other intermediate-potency analgesic agents in children. For these reasons, the most recent WHO guidelines for the management of pain in children with cancer (or other medical illness) are now based on a two-step approach.
The WHO guidelines, which provide an effective, systematic approach to pain that includes strong opioids, have facilitated other WHO initiatives, such as increasing worldwide access to essential medicines for children with painful conditions. The WHO approach, however, may not adequately emphasize a multidisciplinary approach from the start, and thus opportunities to use adjuvant medications (e.g., steroids and local anesthetics) and cognitive or behavioral interventions to reduce pain and pathologic responses to pain may be missed.
Analgesic Medications and Interventions
Nonopioid Analgesics
Nonopioid analgesics are appropriate for mild pain in a child who is not already receiving analgesics. Nonopioid analgesics include acetaminophen, NSAIDs, salicylates, and selective cyclooxygenase (COX-2) inhibitors.
Nonopioid analgesics are frequently used as monotherapy for mild pain and are used together with opioids for more severe pain. Unlike opioids, they do not cause sedation, tolerance, and respiratory depression. When given as an adjuvant to opioids, they enhance analgesia. To this end they may be opioid-sparing (i.e., decreasing the amount of opioid required for adequate analgesia), thereby also limiting opioid-associated adverse effects. A recent randomized double-blind study of infants who underwent noncardiac surgery demonstrated that children randomly assigned to receive intermittent intravenous (IV) paracetamol with morphine as needed for rescue required only about one third as much rescue morphine as children randomly assigned to receive an IV placebo. Combinations of nonopioids and opioids are available, although dosing of such a combination is often limited by the maximum dose of the nonopioid.
Salicylates and NSAIDs
Salicylates and NSAIDs nonspecifically inhibit cyclooxygenase enzymes, thereby blocking production of a variety of prostaglandins that mediate pain, inflammation, fever, and platelet function, protect gastric mucosa, and maintain a physiologic distribution of blood flow in the liver and kidneys. The permanent effects of aspirin on platelet function via permanent acetylation of cyclooxygenase are of particular concern in patients who have thrombocytopenia because the antiplatelet effect lasts even after the drug has been metabolized. Choline magnesium salicylate (Trilisate) is a salicylate that provides many of the same benefits as those provided by other salicylates or NSAIDs without known antiplatelet activity. Salicylates, however, have been associated with Reye syndrome in children younger than 2 years.
No particular NSAID or route of administration has been found to be superior over another. Even ketorolac, the only parenteral NSAID widely used in the United States, is no more effective than orally administered NSAIDs, especially if doses are compared in an equitoxic range. A single NSAID dose is roughly equivalent to 5 to 10 mg of intramuscular morphine in adults and has fewer adverse effects. NSAIDs are commonly regarded as having a ceiling effect with no added benefit at supramaximal doses, although the doses commonly recommended (based on safety concerns) remain well below the ceiling in most cases. NSAIDs can cause nephropathy, gastritis, and bleeding from reversible platelet dysfunction, a characteristic that may limit their use in patients with cancer who are thrombocytopenic. COX-2 inhibitors (e.g., celecoxib) selectively block production of prostaglandins that mediate inflammation and pain without impairing platelet function and with fewer effects on gastric mucosal integrity compared with traditional NSAIDs, particularly with short-term use. Concerns have been raised regarding effects of COX-2 inhibitors on the risk of cardiovascular events in adults. Evidence does not currently indicate that these concerns extend to children with neoplasms, including the small subgroup of children with tumors or vascular anomalies who have an increased risk of thrombotic events.
Acetaminophen
Acetaminophen is a nonopioid analgesic that reduces pain and fever. It may act in part by inhibiting prostaglandin synthesis in the CNS. Unlike salicylates and NSAIDs, which inhibit peripheral cyclooxygenases, it does not have peripheral antiinflammatory properties. Acetaminophen is available in a variety of oral formulations, as an IV formulation (approved in the United States for children ≥2 years), and as a rectal suppository, although rectal administration should be avoided in neutropenic patients. When not contraindicated, rectal administration is helpful for children unwilling or unable to take oral medications, although its absorption is slow and variable, peaking at 70 minutes. Because rectal absorption is less efficient, single doses of 30 to 40 mg/kg can be administered. Subsequent doses should be smaller (20 mg/kg), and the interval between doses should be increased to 6 to 8 hours. Dosing of oral or IV acetaminophen is limited to 75 mg/kg/day or a maximum of 4 g/day (whichever is smaller) because of the risk of hepatic toxicity.
Opioid Analgesics
General Considerations.
Guidelines for initial opioid dosages are presented in Table 71-1 and are discussed in greater detail in this section. Ultimately, the correct dose of opioid is the dose that provides the desired analgesia with acceptable adverse effects. In general, intolerable adverse effects are usually the dose-limiting factor, as opposed to opioids having a ceiling effect. This situation should be explained to parents, as well the fact that, with rare exceptions, children with cancer who receive opioids for pain control do not become addicted to opioids. Addiction is an aberrant psychiatric condition in which the person exhibits maladapted, drug-seeking behavior. True addiction is comparatively rare in patients with cancer-related pain. Children who exhibit exaggerated pain behaviors (e.g., demanding pain medication and engaging in manipulative behaviors) are far more likely to be demonstrating pseudoaddiction, in which case their behavior is a reflection of poorly controlled pain.
| Drug | EQUIANALGESIC DOSES | USUAL STARTING IV OR SUBCUTANEOUS DOSES AND INTERVALS | Ratio of Parenteral to Oral Dose | USUAL STARTING ORAL DOSES AND INTERVALS | |||
|---|---|---|---|---|---|---|---|
| Parenteral | Oral | Child <50 kg | Child ≥50 kg | Child <50 kg | Child ≥50 kg | ||
| Morphine | 10 mg | 30 mg (long term) 60 mg (single dose) | Bolus: 0.1 mg/kg every 2-4 h Infusion: 0.03 mg/kg/h | Bolus: 5-8 mg every 2-4 h Infusion: 1.5 mg/h | 1 : 3 (long term) 1 : 6 (single dose) | Immediate release: 0.3 mg/kg every 3-4 h Sustained release: 20-35 kg: 10-15 mg every 8-12 h 35-50 kg: 15-30 mg every 8-12 h | Immediate release: 5-20 mg every 3-4 h Sustained release: 30-45 mg every 8-12 h |
| Oxycodone | NA | 15-20 mg | NA | NA | NA | 0.1-0.2 mg/kg every 3-4 h | 5-10 mg every 3-4 h |
| Methadone † | 10 mg | 10-20 mg | 0.1 mg/kg every 4-8 h | 5-8 mg every 4-8 h | 1 : 2 | 0.1-0.2 mg/kg every 4-8 h | 5-10 mg every 4-8 h |
| Fentanyl | 100 µg (0.1 mg) | NA | Bolus: 0.5-1.0 µg/kg every 1-2 h Infusion: 0.5-2.0 mg/kg/h | Bolus: 25-50 µg every 1-2 h Infusion: 25-100 µg/h | NA | NA | NA |
| Hydromorphone | 1.5-2 mg | 6-8 mg | Bolus: 0.02 mg/kg every 2-4 h Infusion: 0.006 mg/kg/h | Bolus: 1 mg every 2-4 h Infusion: 0.03 mg/h | 1 : 4 | 0.04-0.08 mg/kg every 3-4 h | 2-4 mg every 3-4 h |
| Meperidine (pethidine) ‡ | 75-100 mg | 300 mg | Bolus: 0.8-1.0 mg/kg every 2-3 h | Bolus: 50-75 mg every 2-3 h | 1 : 4 | 2-3 mg/kg every 3-4 h | 100-150 mg every 3-4 h |
* Doses are for patients older than 6 months of age. In infants younger than 6 months, initial per-kilogram doses should begin at roughly 25% of the per-kilogram doses recommended here. Higher doses are often required for patients receiving mechanical ventilation.
† Use of methadone requires additional vigilance because it can accumulate and produce delayed sedation. If sedation occurs, doses should be withheld until sedation resolves. Thereafter, doses should be substantially reduced; the interval between doses should be extended to 8 to 12 hours, or both.
‡ Use of meperidine, especially long-term use, should generally be avoided if other opioids are available, because its metabolites can cause seizures.
Addiction should be distinguished from dependence, that is, a physiologic response to opioids in which abrupt removal leads the patient to experience symptoms of withdrawal. It may be helpful to draw a parallel to blood pressure medication, the abrupt cessation of which causes an undesired rebound hypertensive effect because the body has adjusted to the presence of the antihypertensive drug. Addiction should also be distinguished from tolerance, which is another physiologic response of the body to the presence of opioids that requires increasing doses to achieve the same analgesic effect. The need to adjust dosing to account for tolerance is not an indication of addiction. Other processes that can resemble tolerance, including opioid-induced hyperalgesia, are discussed later in this chapter.
Opioids do not need to be saved for cases of extreme pain, because increasing pain can often be managed by increasing the opioid dose. In addition, when escalating doses provide marginal benefit, rotating to an alternative opioid may provide better analgesia. It may be helpful to explain to parents that good pain control from the start may improve pain control overall and actually minimize the amount of opioid that is ultimately needed because when good pain control is achieved, the need to use large doses required to catch up to uncontrolled pain can be avoided.
Some children who have cancer, particularly advanced cancer, require extremely high doses of opioids. For example, in a sample of children with advanced cancer, Collins and colleagues found that in some patients the opioid infusions ranged more than 100-fold from 3.8 to 518 mg per hour of morphine equivalent.
Some studies conducted in vitro and in animal models have demonstrated that opioids may promote cancer cell growth by affecting processes such as tumor cell proliferation and migration. For example, opioids at physiologically relevant concentrations promote tumor angiogenesis. In addition, the opioid receptor antagonist naloxone and the COX-2 inhibitor celecoxib inhibit angiogenesis, tumor growth, and metastasis in rodents. However, other studies demonstrate growth-inhibiting effects of opioids and have been reviewed elsewhere. No evidence to date demonstrates opioid-associated promotion of tumor growth in humans. These preliminary findings should not, by themselves, lead to the conclusion that use of opioids to relieve cancer-related pain should be avoided. The data accumulated to date regarding the potential risks of opioids and the benefits of adequate analgesia are insufficient to recommend limiting opioid use for analgesia.
Developmental Pharmacology.
Elements of renal clearance such as glomerular filtration and tubular secretion increase in the first few weeks of life, such that renal clearance commensurate with adult clearance is achieved by 8 months. When compared with children and adults, neonates and infants have reduced hepatic clearance as a result of hepatic enzyme immaturity. In addition, children 2 to 6 years of age have higher clearance than do adults because of a larger liver mass to body weight ratio. As a result, drugs may need to be administered more frequently in children than they are in adults. Other age-related differences, such as changes in body composition and plasma concentrations of drug-binding proteins, can also influence pharmacokinetics.
Choice of Opioid.
The usual starting opioid is morphine because of its low cost, wide availability, multiple routes of administration, and familiarity to clinicians. Because of its long history of use in children, it should be considered the first-line opioid in this population unless specific reasons exist to consider alternatives. Alternative opioids including oxycodone, hydromorphone, and semisynthetic and synthetic compounds such as fentanyl and methadone can be used in children. Use of alternative opioids may be predicated on availability, route of administration, presence of organ impairment, and the patient’s prior experience with particular opioids.
Renal Failure.
Metabolites of morphine, oxycodone, and hydromorphone (discussed later) may accumulate during renal failure. Some metabolites have analgesic activity, which may lead to delayed opioid toxicity (opioid neurotoxicity will be discussed later). For this reason, dosing intervals may need to be increased in patients with renal dysfunction. The pharmacokinetics of alternative opioids such as fentanyl and methadone are not changed with renal impairment, making these opioids better choices in this setting.
Hepatic Failure.
Because glucuronidation is relatively well preserved in persons with liver failure, opioids metabolized by glucuronidation (i.e., morphine, hydromorphone, and buprenorphine) are generally better choices than those metabolized by oxidation via liver cytochromes (e.g., oxycodone, fentanyl, and methadone) in this setting. However, because of shunting in persons with liver cirrhosis, the bioavailability of glucuronidated opioids may be increased. For this reason, initial opioid doses should be lower in persons with hepatic impairment.
Weak Opioids.
Tramadol is an atypical analgesic with some direct noradrenergic and serotonergic agonist action and with an active metabolite that is a weak opioid. Tramadol has numerous drug-to-drug interactions that should be considered. For example, it can produce seizures by itself, but this risk is greatly increased when tramadol is administered in combination with several classes of antidepressants. It is available in oral immediate and extended-release preparations and in combination formulations with acetaminophen.
Although codeine has historically been recommended as a weak opioid for mild to moderate pain, it has several limitations. The variable expression of the CYP2D6 enzyme that biotransforms codeine leads to unpredictable levels of the active metabolite, morphine. In the fetus, CYP2D6 activity is absent, and in children younger than 5 years, CYP2D6 activity is less than 25% that of adult activity. Furthermore, certain genotypes are associated with reduced enzyme activity, regardless of age. In a study of 96 children randomly assigned to receive either morphine and diclofenac or codeine and diclofenac after an adenotonsillectomy, it was found that 47% of children had genotypes associated with reduced enzyme activity and that neither morphine nor its metabolites were detected in 36% of children with reduced CYP2D6 activity genotypes who received codeine, although the study did not account for other factors influencing catalytic rate, such as gene copy number. Such “poor metabolizers” of codeine were more likely to have uncontrolled pain and to require rescue medication.
On the other hand, persons who are “ultrarapid metabolizers” may possess multiple copies of the CYP2D6 gene responsible for codeine metabolism or have genotypes associated with rapid metabolism and may therefore be at risk for adverse effects such as respiratory depression from rapid generation of morphine from codeine. Several pediatric deaths from codeine administered after a tonsillectomy and/or an adenoidectomy have resulted from such ultrarapid metabolism. It is for this reason that the U.S. Food and Drug Administration (FDA) has issued a “boxed warning” and deemed codeine contraindicated in this setting.
In a variety of painful conditions in children, provision of codeine provides no benefit greater than that of NSAIDs. When codeine, ibuprofen, and acetaminophen monotherapy were compared in children with musculoskeletal trauma who presented to an emergency department, ibuprofen provided superior analgesia. In the posttonsillectomy setting, codeine in combination with acetaminophen caused more nausea with no difference in pain or postoperative bleeding. A meta-analysis also found that weak opioids (i.e., codeine) in combination with NSAIDs fail to provide superior analgesia to that provided by NSAIDs alone but do have significantly more adverse effects. Based on these findings, in our view, very few instances exist in which codeine is a preferred choice among opioids.
Some opioids exhibit partial mu receptor agonist activity (e.g., buprenorphine), kappa agonist activity (e.g., nalbuphine), or mixed agonist activity (e.g., butorphanol and pentazocine). Although these opioids have predominantly agonist activity, some have significant antagonist activity as well, and thus they may reduce the effect of pure mu agonists given concurrently. In general they do not provide superior analgesia, although they may have fewer gastrointestinal (GI) or respiratory adverse effects and may be considered for individual patients who have limiting adverse effects with other opioids. Currently the greatest use of buprenorphine in the United States is for substance abuse treatment. It has been used by multiple routes for treatment of cancer-related pain in children, particularly in countries with greater impediments to the prescribing of morphine, methadone, or other opioids. Authors of a recent small prospective case series reported reasonable effectiveness and tolerability of transdermal buprenorphine for children with cancer.
Strong Opioids.
Meperidine is a strong opioid that should be avoided in most cases because of its adverse effect profile and lack of superiority to the strong opioids described in the next sections. For example, repeated doses of meperidine lead to accumulation of its metabolite, normeperidine, which in turn causes neuroexcitatory symptoms including agitation, tremors, myoclonus, and seizures. In a double-blind trial comparing morphine with meperidine administered via patient-controlled analgesia (PCA), it was found that morphine resulted in better analgesia and had no more adverse effects than did meperidine. In low doses, meperidine reduces postoperative shivering or rigors associated with amphotericin infusion.
Morphine.
Morphine is the most frequently prescribed opioid in children and is the best studied opioid in this population. It offers flexibility in terms of routes of administration. It is also available in a controlled-release formulation, and multiple randomized controlled trials have shown that this formulation can effectively control cancer-related pain when administered every 12 hours. Morphine is extensively metabolized by glucuronidation in the liver to morphine 3-glucuronide (M3G) and morphine 6-glucuronide (M6G). M3G does not bind mu receptors and has no analgesic activity but may contribute to some of the neuroexcitatory adverse effects of morphine. On the other hand,M6G does bind mu receptors and is a potent analgesic.
Oxycodone.
Oxycodone is a semisynthetic derivative of morphine. Although it is frequently categorized as a weak opioid appropriate for mild to moderate pain (and is frequently administered in combination with acetaminophen), this categorization is a reflection of its use at low doses. In fact, a meta-analysis showed that oxycodone is as efficacious an analgesic as morphine or hydromorphone. This meta-analysis also showed no difference between oxycodone and morphine in terms of adverse effects such as dry mouth, sedation, or nausea. Oxycodone itself has no ceiling effect or dose limit, although dosing may be limited when it is administered in combination with nonopioid agents such as acetaminophen. Although it is available in parenteral formulations in other countries, in the United States it is only available in an oral formulation. The oral formulation is available in an extended-release preparation.
Oxycodone is predominantly metabolized by CYP3A4 to inactive products. A secondary pathway involving CYP2D6 may lead to generation of the active opioid oxymorphone in patients with ultrarapid metabolizing variants. Although cases of apnea and death associated with excessive conversion from oxycodone to oxymorphone have been published, the prevailing impression is that pharmacogenomic variation overall leads to less variance in effect for oxycodone compared with codeine. Oxycodone pharmacokinetics have been studied in children, but there is a need for additional pediatric pharmacokinetic/pharmacodynamic studies that include oxymorphone assay, pharmacodynamic end points, and contemporary genotyping.
Hydromorphone.
Hydromorphone may be administered orally, intravenously, or subcutaneously. Although it was previously thought that hydromorphone has less neurotoxicity, hydromorphone metabolites have recently been shown to convey neuroexcitatory adverse effects. One advantage of hydromorphone compared with morphine is that its higher potency allows for smaller subcutaneous volumes to be delivered when this route of administration is utilized.
Fentanyl.
Fentanyl may be given intravenously with rapid onset of action and short duration of action (20 to 30 minutes). For this reason fentanyl is frequently used as an analgesic for brief, painful procedures. Rapid administration of fentanyl may cause chest wall rigidity that requires reversal with naloxone or even neuromuscular blockade and positive pressure ventilation. In occasional patients, fentanyl may be better tolerated than other opioids, in part because it is associated with less histamine release and creates no metabolites that may produce neurotoxicity.
Fentanyl transdermal patches last 72 hours and are a convenient parenteral mode of drug delivery that is preferred by many adults. These patches have also been used successfully in children who have chronic pain. Although wide within-individual variability in fentanyl absorption exists, intraindividual absorption is reported to be stable. In addition, hyperhidrosis, hypertrichosis, and the localization of patches on the skin do not appear to affect fentanyl absorption. Because the onset of action is at least 12 hours, and because some fentanyl remains in the system for 72 hours after patch removal, transdermal fentanyl lacks flexibility for close titration for rapidly changing pain severity. The smallest patch size (12 µg/h) may be too large a dose for some children. For children as young as 2 years who had previously taken opioids and had developed some degree of tolerance, transdermal fentanyl was found to be a safe and well-tolerated alternative to oral opioid treatment. The reservoir design of the patch prevents the patch from being cut to adjust the dose delivered. Transdermal fentanyl should be avoided in patients who have not previously taken opioids because it may result in respiratory depression.
In addition to rapid onset of action and transdermal application, fentanyl provides several other benefits. When renal function is impaired, fentanyl does not accumulate to the same extent as morphine. Some studies have demonstrated that transdermal fentanyl appears to cause less constipation than does oral morphine, but it is unknown whether this observation is related to the route or the drug.
In adults or children already receiving 60 mg/day of morphine, oral transmucosal fentanyl citrate (OTFC, or Actiq) provides extremely rapid control of incident pain, with an onset of action of 5 to 10 minutes. This rapid onset of action is due to its lipophilic nature, as well as because this route bypasses first-pass hepatic metabolism. Because of the rapidity of its onset of action, OTFC has been used to provide analgesia for brief, painful procedures without the requirement for IV access. OTFC is also quite useful for patients who have breakthrough pain. In a double-blind, double-dummy, randomized, crossover study of adult patients with cancer who had incident (breakthrough) pain, it was found that OTFC reduced pain intensity more effectively than did immediate-release oral morphine, and OTFC was favored over immediate-release morphine sulfate by more patients after the study. No conversion ratio is available for OTFC, and careful titration is needed to determine the correct dose. No correlation exists between the effective OTFC dose and the around-the-clock dose of an opioid. For this reason the lowest strength (200 µg) should be tried first. If inadequate pain relief is achieved in 20 minutes, this dose may be repeated.
The fentanyl buccal tablet is another preparation of fentanyl that is rapidly absorbed through effervescent action through the oral mucosa. Patients should be taking at least 60 mg/day of oral morphine so they have opioid tolerance to safely receive a fixed dose of fentanyl with such rapid onset of action through the oral mucosa. The need for this degree of tolerance is highlighted by recent experiences with buccal/sublingual fentanyl tablets, which, when (inappropriately) administered to opioid-naive patients, may result in life-threatening respiratory depression.
Methadone.
Methadone may be given orally (it is available as a tablet or liquid) or intravenously. One advantage to methadone in the pediatric population is that it is the only long-acting opioid widely available in liquid formulation. In addition, it is relatively inexpensive to manufacture, costing 90% less than extended-release morphine. Methadone also exhibits unique receptor-binding properties in that the l-isomer binds mu opioid receptors and the d-isomer binds the N-methyl-d-aspartate (NMDA) receptor. Because the NMDA receptor is involved in opioid tolerance, opioid hyperalgesia, and neuropathic pain, it may be a useful opioid in these clinical situations, which are discussed later. For these reasons, administration of methadone for analgesic purposes has become more popular in recent years.
The variable pharmacologic half-life of methadone, which ranges from 12 to 150 hours, may result in delayed toxicity (e.g., sedation and hypoventilation) that can occur many days after initiation of the drug. The analgesic half-life of methadone is commonly cited as 4 to 6 hours, although some patients can require minimal rescue analgesia with dosing at 8- or 12-hour intervals. In addition, equianalgesic dose conversion from other opioids is variable and depends in part on the dose of the previous opioid. Its potency relative to morphine is highly dependent on the previous morphine dose. A very convenient website, www.globalrph.com/narcoticonv.htm , can be used to assist in dose conversions. It should be emphasized that even with these calculations, enormous individual variability exists, and close follow-up is required to avoid delayed oversedation.
Methadone is metabolized through several cytochromes and therefore interacts with a variety of other medications. Individual variation in cytochrome expression may account in part for significant blood concentration variability in patients. Methadone, in conjunction with other medications, may prolong the QTc interval. It is unclear whether this phenomenon explains the otherwise unexplained increased incidence of sudden cardiac arrest in adults receiving methadone therapy. Until the potential for methadone to pose cardiotoxicity is better understood, it should be used cautiously in children who have underlying cardiac conditions or those at risk for prolonged QTc. Although methadone has several advantages, it also has some unique features that require familiarity with this agent for safe and effective use. The majority of pediatric oncologists rarely, if ever, prescribe methadone. Lack of familiarity with methadone pharmacodynamics, effectiveness, and dosing equivalence are the most common reasons cited for prescribing other opioids rather than methadone.
Oxymorphone.
Oxymorphone is the active metabolite of oxycodone and is available as a rectal suppository. An extended-release oral formulation has also recently been approved.
Opioid Starting Doses.
For the opioid-naive patient, recommended starting doses are listed in Table 71-1 . For infants younger than 6 months, the starting dose should be roughly one fourth the weight-scaled dose suggested in the table and titrated to effect. Opioids should be administered with caution in patients who have disordered control of respiration, altered mental status, or altered drug metabolism. This is not to say, however, that opioids should be withheld from these patients. In fact, opioids can be safely delivered and adequate analgesia achieved with careful titration to effect.
Routes and Methods of Administration.
Medication should be given by the simplest, most effective, and least distressing route. Other considerations that should guide the choice of route include the severity and type of pain, the ability of the child to tolerate a given route due to developmental or personal factors, and the ability of the caregivers to administer medication via certain routes.
Oral.
When possible, the oral route of administration should be attempted first. In general, the time for opioids to reach peak effect is about 60 minutes with the oral route. Most extended-release preparations are available in tablets or capsules. For children who cannot swallow tablets or capsules but who would benefit from an extended-action preparation or agent, liquid methadone can be used. If the child has a gastrostomy tube, ultra–extended-release morphine (given every 24 hours) may be suspended (but not crushed) and administered via a gastrostomy tube. Ultra–extended-release morphine allows once-daily oral dosing, but unintended chewing or crushing may lead to overdose from immediate release of the morphine. Opening the capsules and sprinkling the drug onto applesauce may be an appropriate administration technique for adults but should be avoided in young children. Ultra–extended-release morphine may be considered for adolescents, but in younger children who may be at risk for accidentally chewing the capsules, these preparations should generally be avoided.
For intermittent dosing, oral opioids prepared as concentrated drops may provide analgesia without the requirement of swallowing larger volumes of liquid. Concentrated morphine may be helpful for children who are unable to reliably take oral opioids because of their neurodevelopmental capacity or nausea and vomiting.
Rectal.
Suppositories containing hydromorphone and morphine may be administered rectally. In addition, controlled-release morphine tablets may be given rectally. Although the published potency of rectal opioids approximates that of oral opioids, the pharmacokinetic properties of morphine, that is, first-pass metabolism to the active metabolite M6G by the portal circulation, should be considered when considering rectal administration. For example, Wilkinson and colleagues determined that the area under concentration-time curves for morphine metabolites were approximately twice those achieved after rectal administration. The maximal concentration of morphine and its metabolites was lower and the time to achieve peak levels was longer for rectal administration. In this study, the variation in morphine kinetics did not correspond with altered pain ratings. Although it is reasonable to start with 1 : 1 (oral to rectal) equianalgesic dosing of morphine, adjustments in the dose or dosing interval should be anticipated.
Transdermal.
Fentanyl and buprenorphine are the only opioids manufactured in transdermal formulations. The transdermal route for delivery of fentanyl or buprenorphine provides some advantages. Other opioids such as morphine may be compounded as transdermal formulations, but absorption of these other opioids via this route is unreliable, and alternative means of opioid administration are almost invariably available.
Intravenous.
Use of the IV route, when appropriate, may provide rapid and reliable administration. In general, the time for morphine to reach its peak effect is about 15 minutes via this route. The IV route is frequently used for patients who have severe pain or for children who are unable to take oral medications, such as children who are in the final stages of life.
Subcutaneous.
All IV opioids may be administered subcutaneously, although methadone may cause local irritation if infused continuously. Delivery of opioids by this route dose adds approximately 30 minutes to the time of peak effect obtained by IV administration. This route is simple to use and requires only a portable syringe driver to administer the medication through a butterfly needle. In addition, it confers consistent delivery and easy titration without the requirement for IV access. Needles are changed every week or more often if skin irritation occurs, and this task can often be performed by a family member. In our experience, children can absorb 2 mL per hour, whereas adults can absorb 3 to 5 mL per hour. Higher doses of opioids may exceed these volume limits. In such cases, switching to a more potent opioid, such as hydromorphone, may be necessary.
Patient-Controlled Analgesia.
PCA can provide a continuous IV or subcutaneous infusion of opioid to provide basal control of pain, as well as a bolus, which provides relief from breakthrough pain. The PCA delivery system allows patients to manage their pain themselves, and no lag time exists between the request and delivery of a bolus dose for uncontrolled pain, increasing their sense of control over their pain. In one study comparing continuous infusion morphine with PCA, it was found that patients who used PCA required less total opioid while receiving equivalent control of the pain of mucositis associated with bone marrow transplantation.
Because opioid-induced sedation generally occurs before respiratory depression, it is rare for a patient to administer boluses to himself or herself to the point of respiratory depression. PCA delivery has been used in the pediatric population with safety and efficacy. PCA does not increase the incidence of opioid-related complications, including sedation. Clinical protocols for calculating the PCA commencement opioid dose and subsequent opioid-dose escalation can facilitate the safe and efficacious implementation of PCA. Thorough assessment of PCA use includes the total daily dose, ratio between continuous and bolus opioid, amount of baseline and breakthrough pain, and response to the bolus dose. A common recommendation is to adjust the continuous (basal) rate to supply roughly two thirds of the patient’s daily opioid requirement. Although this starting point is reasonable for most oncology patients with persistent disease-related pain, individual circumstances exist in which the parameters should be modified. For example, in the setting of brief, severe, episodic pain, it may be reasonable to use a lower basal rate and give more generous boluses. In postoperative care, considerable variation exists in the use of basal infusions. Our practice is to use them for operations expected to result in more severe pain and/or in patients who are likely to underdose themselves. Conversely, we tend to avoid basal infusions or use very low basal rates for patients who have less painful surgery, for patients who have received other nonopioid methods of analgesia (e.g., peripheral nerve blocks or plexus blocks), or for patients who have factors that increase respiratory risks.
PCA has been used successfully in children as young as 4 years of age. PCA may also be administered by surrogates, commonly as nurse-controlled analgesia or parent-controlled analgesia, or collectively as PCA-by-proxy. In theory the safety of PCA is maximized when the patient self-administers the medication, because when the patient falls asleep, he or she stops pushing the button. Because the proxy assesses the child’s pain and provides the bolus dose, the safety feature inherent in a PCA to prevent respiratory depression is overridden, but when it is used appropriately it is associated with only rare respiratory or neurologic complications. Overall, the safety of nurse-controlled analgesia is well established and is widely used for both opioid-tolerant and opioid-naive infants and children. Greater controversy persists with parent-controlled PCA, although experience with this arrangement is mounting. Our general practice is to greatly limit its use for routine care of opioid-naive postoperative infants and children but to use it widely for opioid-tolerant infants and children in palliative care, especially at home. Guidelines to increase the safety of PCA by proxy have been proposed.
Intrathecal.
Opioids may be administered intrathecally and are considered later in the “Invasive Approaches to Pain Management” section.
Regardless of the route utilized, if the patient has continuous pain, a regimen providing continuous pain control should be implemented. To establish a patient’s true analgesic needs, short-acting opioids, which can be easily titrated, may be administered for the first 24 to 48 hours. Based on the amount of opioid required during the initial interval, a longer acting opioid may then be substituted, with provision of an as-needed short-acting opioid available for incident or breakthrough pain.
Whatever the regimen, ensuring proper follow-up assessment is critical to ensure appropriate analgesia and to evaluate for potential opioid-associated adverse effects. Because constipation is a predicted and preventable adverse effect of opioids, a bowel regimen to prevent this adverse effect should always be instituted and adjusted as necessary whenever opioids are started or escalated.
Breakthrough Pain.
Breakthrough pain is a transitory exacerbation of pain that occurs when pain is otherwise relatively controlled or stable at baseline. Patients with chronic cancer-related pain and superimposed breakthrough pain have worse overall pain, more impaired functioning, and higher psychological distress than do patients with chronic cancer-related pain alone. Breakthrough pain may occur as a result of incident pain (i.e., pain due to a stimulus, such as movement or coughing) or end-of-dose failure, or it may be spontaneous in nature (such as lancinating pain attacks in persons with postherpetic neuralgia).
In a prospective study of pediatric inpatients with cancer it was found that 57% experienced one or more episodes of breakthrough pain during the preceding 24 hours, with each episode lasting seconds to minutes and most commonly characterized by the children as “sharp” and “shooting.”
Breakthrough pain may be challenging in that its onset may be unpredictable and rapid, and it may be more severe than pain typically experienced at baseline. For these reasons, use of a pain diary may be particularly important in detecting patterns and factors associated with breakthrough pain. If end-of-dose breakthrough pain occurs, the total daily around-the-clock dose may be increased by 25%, although this increase may lead to intolerable adverse effects. Alternatively, the dosing interval may be shortened.
Breakthrough pain should be approached as other types of pain are approached, with (1) consideration of the underlying triggers and interventions aimed at the underlying problem, (2) optimization of the scheduled analgesic regimen, and (3) use of adequate analgesics for episodic pain, that is, rescue medication. The rescue dose to treat such breakthrough pain should be the equivalent to the dose used every 4 hours, or 5% to 10% of the total daily opioid dose. For predictable incident pain, a short-acting opioid given just prior to the activity may be helpful. When the breakthrough pain has neuropathic features, consideration should be given to adjunctive use of analgesics with specificity for these types of pain, that is, anticonvulsants or antidepressants, as will be discussed later.
OTFC may safely provide very rapid pain relief in patients receiving the equivalent of 60 mg/day of oral morphine, as was previously discussed. It may be especially useful when breakthrough pain is unpredictable and short-lived. Other hydrophilic agents such as morphine are often administered sublingually for breakthrough pain but have a longer onset of action. A recent small study indicates that the rapid onset of action of methadone may make it a useful agent for treatment of breakthrough pain.
Dose Escalation.
When a patient who is already receiving oral or IV opioids has severe pain that persists despite a dose of breakthrough opioid medication, the dose should be increased by 50% to 100% and repeated. Once adequate analgesia is reached, the total amount given over 4 hours to determine the “effective dose” for every 4 hours should be calculated. If more than two rescue doses are used in a 24-hour period, the total standing dose should be increased.
For patients receiving continuous IV opioids, a rescue dose for breakthrough pain should be provided that consists of 50% to 200% of the hourly infusion every 15 minutes as needed. For the sake of simplicity, use of multiple different short-acting opioids is discouraged.
Opioid Tolerance.
The response to acquired tolerance to an opioid is usually to increase the opioid dose. When this approach is not feasible because of opioid adverse effects such as neuroexcitability, rotating to a different opioid may be an option. Because the NMDA receptor plays a role in opioid tolerance, methadone, with its attendant NMDA antagonist properties, may control pain that is otherwise refractory to opioids. The addition of adjuvant NMDA receptor antagonists such as ketamine may also reverse opioid tolerance. In animals, as well as in some patients, chronic administration of opioids can generate a condition of generalized hyperalgesia, meaning that new painful stimuli produce a greater intensity of pain than would have occurred with the same stimulus for that subject in an opioid-naive state. Opioid-induced hyperalgesia is discussed in greater detail later.
Opioid Rotation.
Opioid rotation, or switching, may also be indicated as a result of intolerable adverse effects or route of administration. Equianalgesic tables are useful for conversion from one opioid to another and are widely available. Although no evidence exists to demonstrate the superiority of one opioid over another in such a switch, the strategy of changing opioids may be successful in alleviating dose-limiting adverse effects or opioid tolerance in children. For example, Drake and colleagues found that opioid rotation resolved adverse effects in 90% of children without loss of analgesia or the need to increase morphine equivalents. In fact, because of the phenomenon of incomplete tolerance, the equianalgesic dose of the second opioid should be decreased by 20% when a patient is transitioned from one opioid to another. The phenomenon of incomplete tolerance is particularly pronounced when converting to methadone, likely because of the ability of the d-isomer of methadone to act as an NMDA receptor antagonist.
Opioid Withdrawal and Opioid Tapering.
Opioid withdrawal is a physiologic response. Sudden discontinuation of opioids in a patient who has been taking long-standing opioids may prompt a withdrawal syndrome characterized by irritability, restlessness, dysphoria, anxiety, muscle aches, sweating, piloerection, diarrhea, nausea, vomiting, yawning, and sneezing. Withdrawal may be prevented by tapering opioids. Opioid doses may be safely cut in half without precipitating withdrawal. Continued tapering may be achieved by halving the dose every 3 days. An alternative strategy is to reduce the total daily dose by 10% per day. Maintaining the rescue dose at its original dose during the tapering process allows for effective treatment of breakthrough pain that may occur as the dose is tapered.
Opioid Adverse Effects.
Nonrespiratory adverse effects from opioids include constipation, nausea, pruritus, somnolence/sedation, and, particularly at high doses, neurotoxicity. A prospective study of pediatric oncology patients found that in children receiving morphine, postoperatively, 38% had vomiting, 32% had nausea, and 24% had constipation. The high incidence of nausea and vomiting in this population is likely confounded by postoperative nausea and vomiting.
Opioid-associated sedation typically self-resolves within a few days of the institution of opioids. In some cases the sedation observed when instituting opioids is not an adverse effect of opioids per se but rather a result of an exhausted patient finally able to sleep once pain is controlled. Nausea experienced by some patients upon starting opioids similarly self-resolves within a few days and is often relieved with use of an antiemetic. Switching to a different opioid due to nausea within the first 3 days of opioid therapy may be premature. Opioid-associated urinary retention is an uncomfortable symptom that may respond to a switch to an alternative opioid. Anecdotally, urinary retention in some patients may respond to selective alpha-1A antagonists such as tamsulosin, which are commonly prescribed for adults with lower urinary tract outflow obstruction. Urinary retention, frequency, or urgency should prompt a focused consideration of a range of oncologic and neurologic causes, as well as effects of other medications in addition to opioids, such as anticholinergics and antihistamines. Management of opioid-associated sedation/fatigue, constipation, pruritus, and nausea are further discussed in the sections of this chapter dedicated to these specific symptoms.
If adverse effects persist despite appropriate interventions, rotation to a different opioid may be helpful. Overall, at this point no clear evidence exists that one particular opioid has a different adverse effect profile than another opioid. Patients may exhibit different sensitivities to different opioids because of individual variability in opioid receptors, pharmacokinetics, and metabolism. Inroads have recently been made in understanding which genetic variants may influence a particular person’s response to opioids. For example, certain variants of the multidrug resistance 1 gene (formerly MDR1, now called ABCB1 ) or the gene encoding catechol-O-methyltransferase (COMT) are associated with CNS adverse effects, such as drowsiness or confusion. A very promising recent approach to reducing opioid adverse effects involves identifying opioid agonists with “ligand biasing” of actions on opioid receptors that preferentially act via G proteins rather than via beta-arrestin signaling.
Opioid-associated adverse effects may be also improved with a reduction in opioid dose, which may be achieved without loss of analgesic effect if a coanalgesic medication is added. For example, when gabapentin and morphine are combined they provide better analgesia from neuropathic pain at lower doses of each drug than either does as a single agent.
Opioid-Induced Neurotoxicity.
Opioid-induced neurotoxicity is typically encountered when opioid doses are rapidly increased, when high doses of an opioid are used, or in the setting of renal failure. The patient may describe increased sensitivity to pain (hyperalgesia), pain in response to nonpainful stimuli (allodynia), worsening pain despite increasing doses of opioids, or pain that appears to spread. Other findings of neurologic hyperexcitability such as myoclonus, delirium, or seizures may also be present. Opioid-induced hyperalgesia is likely due to accumulation of neurotoxic opioid metabolites such as M3G or hydromorphone-3-glucuronide. Such metabolites activate presynaptic calcium channels that release glutamate, which in turn activates NMDA receptors and depolarized postsynaptic neurons in the CNS.
If signs or symptoms of opioid neurotoxicity develop, the opioid should be decreased or changed to one with potentially less neurotoxic effects, such as fentanyl or methadone. The addition of a nonopioid analgesic may lessen the amount of opioid required. An alternative mode of analgesia such as intrathecal, regional, or local analgesia may be used in place of systemic opioids. If needed, parenteral ketamine, an NMDA receptor antagonist, may also be initiated to reduce NMDA receptor-mediated neurotoxicity.
Myoclonus is frequently relieved by a benzodiazepine such as diazepam. Opioids may also play a role in the development of delirium; the reader is referred to the section in this chapter dedicated to mental status changes.
Management of Opioid Overdose.
Respiratory depression occurs as a result of opioid receptor blockade of CO 2 chemoreceptors in the medulla. The risk of respiratory depression from opioids is indeed small when opioids are dosed and titrated judiciously. Scenarios in which opioids may cause respiratory depression are the development of renal failure or a sudden decrease in pain, such as after a neurolytic block if opioid doses are not adjusted.
Respiratory depression characterized by hypopnea alone is not in and of itself an indication for opioid reversal with naloxone. In fact, administration of oxygen and reduction of the subsequent dose may be the only actions needed. For a true respiratory emergency, naloxone should be administered by (1) diluting the 0.4 mg/mL ampule in 10 mL saline solution and (2) administering 1 mL IV or subcutaneously every 3 minutes until respiratory depression improves. If the patient is taking long-acting opioids, repeated doses of naloxone or a naloxone infusion may be required, with the hourly dose being the dose initially required to overcome respiratory depression. Overadministration of naloxone will block opioid receptors, resulting in withdrawal that may be characterized by severe pain and sympathetic instability.
Adjuvant Treatments
Radiotherapy
Radiotherapy is commonly used to relieve pain such as localized bone metastases. In a review of 13 randomized trials in adults with painful bone metastases who received either radiotherapy or radioisotope injection for pain, it was found that 27% achieved total pain relief and 42% attained a 50% level of pain relief. The largest trial found that the median duration of complete pain relief was 12 weeks. A variety of fractionation schedules were used, and no clear difference between schedules was found.
Antidepressants
TCAs and serotonin and norepinephrine reuptake inhibitors (e.g., duloxetine) may relieve neuropathic pain and are discussed in more detail later.
Steroids
Pain attributable to swelling, such as headache from increased intracranial pressure, bone pain, nerve pain from compression by a tumor, and hepatic capsular pain, may be responsive to steroids. A common starting dose is 10 mg followed by 4 mg four times a day in larger patients. Dosages of 10 mg/m 2 followed by 4 mg/m 2 four times a day can be used in smaller patients. If the response is favorable, the steroid dose should gradually be reduced to the lowest effective dose. A medication to provide concurrent gastric protection should also be prescribed.
Anticonvulsant Agents
Anticonvulsant agents control neuropathic pain by preventing peripheral nerve excitation. Carbamazepine has been widely studied for neuropathic pain such as trigeminal neuralgia. However, carbamazepine interacts with many other drugs and may suppress bone marrow function, limiting its usefulness in patients with cancer. Oxcarbazepine is a carbamazepine derivative that acts via use-dependent blockade of sodium channels. Overall, compared with carbamazepine, oxcarbazepine appears to result in fewer risks for hematologic, dermatologic, and hepatic complications. Hyponatremia can occur, and electrolytes should be monitored in the first weeks of therapy or with dose escalation. Gabapentin and pregabalin are newer anticonvulsants that lack such interactions and bone marrow toxicity. These agents target both excitatory and inhibitory neurotransmitters through inhibition of sodium and voltage-gated calcium channels. Gabapentin may be sedating, requiring gradual initiation (i.e., a 5 to 7 mg/kg/dose by mouth three times a day, with a gradual increase every 3 days) and titration to effect. Reports of pediatric uses of gabapentin and pregabalin for treatment of chronic neuropathic pain are limited to case series. For any of the anticonvulsants, effects on mood, sedation, and mental clarity are quite variable.
Clonidine
Clonidine is an alpha-agonist drug that historically has been used to treat hypertension or opioid withdrawal. It may also be used for neuropathic pain or to enhance analgesia from opioids. Clonidine may be administered orally or transdermally, which is an additional benefit in the pediatric population. Clonidine has also been used in children for hyperactivity or steroid-associated psychiatric symptoms. Sedation is common.
Ketamine
Ketamine is an effective adjuvant analgesic that is useful for its opioid-sparing and opioid-sensitizing capabilities, particularly in persons experiencing difficult pain syndromes such as neuropathic pain. Although the evidence is scant, reports exist of its usefulness in relieving refractory pain in children with cancer. Ketamine may on occasion cause hallucinations and depersonalization/derealization. Such effects are more common in adults and may be prevented or treated with a benzodiazepine.
Invasive Approaches to Pain Management
In a small subgroup of patients, aggressive titration of analgesics and adjuvants still results in a circumstance of inadequate analgesia and/or intolerable adverse effects, including intolerable sedation. In some situations consideration of regional anesthetic approaches may be appropriate; the most common approaches are infusions of analgesics and local anesthetics via indwelling intrathecal, epidural, or plexus catheters. A number of considerations influence the choice of these methods, including the wishes of the patient and his or her surrogate in terms of balancing analgesia, alertness, and the risks and inconveniences of a technologic approach; availability of expert personnel for both catheter placement and ongoing management; and consideration of the nature of the pain, the expected course of the disease, and disease-related symptoms. Because a number of technical and management issues cannot be readily extrapolated from similar infusions in adults or from perioperative infusions in children, consultation with clinicians who have experience in these techniques is encouraged. In adults, a track record exists of using implantable programmable pumps that can be refilled at a frequency ranging from weekly to every several months. One study indicated benefits of these systems with regard to pain, quality of life scores, and even longevity.
In children with cancer, it is generally necessary to combine small amounts of a local anesthetic along with opioids in the spinal infusion. Because of the relatively low potency and low solubility of existing local anesthetics, this requirement makes it impractical to use the programmable pumps commonly used in adults. Therefore in a majority of these cases, our preference has been to use implanted intrathecal ports rather than implanted pumps. The procedure to place these ports is performed in the operating room with use of general anesthesia while the patient is in the lateral position. The intrathecal catheters are advanced cephalad from spinal entry near L3-L4 under fluoroscopic guidance to position the tips near the spinal dorsal horn levels (rather than the exiting nerve root levels) relevant to the sites of greatest pain. The reader is referred to Figure 564 in the atlas by Clemente for illustration of the spinal dorsal horn levels.
As an example, for refractory pain due to pelvic or lower extremity tumors predominantly involving lumbosacral dermatomes, we advance the catheter tips to a level around T11. Catheters are tunneled subcutaneously and connected to a port that is positioned over the lower ribs near the anterior axillary line.
In the setting of refractory pain due to advanced cancer, our view is that coagulopathy in most cases should not be regarded as a major contraindication to this procedure. If necessary, platelets and/or fresh-frozen plasma are infused immediately before and during the procedure as guided by coagulation parameters.
Use of indwelling ports is preferable for the sake of sterility and skin care. Ports are accessed with Huber needles similar to those used for IV ports. Dilute local anesthetics are generally required for adequate analgesia (particularly with movement), and they can generally be titrated in a manner that preserves reasonable lower body motor functioning. Depending on the adverse-effect profile seen with opioids and local anesthetics, other spinal analgesics may be included in the mixture, including clonidine and ketamine.
Although the epidural route can be used, our experience is that the intrathecal route is more reliable over a longer time frame because of several factors, including the development of adhesions or fibrosis in the epidural space over periods of months with use of high-concentration infusions and because of the phenomenon of tolerance to local anesthetics, which can be overcome very readily with intrathecal dosing but with much greater difficulty with prolonged epidural dosing. Clinicians’ estimates of prognosis and longevity are often imprecise. Our general recommendation is that, if invasive approaches are being considered in the first place, the most versatile system available should be chosen to be implanted—that is, one most likely to work successfully for the short term (i.e., a few weeks) as well as longer term (i.e., months to a year) and least likely to require a repeat procedure. One potential early complication of this procedure is cerebrospinal fluid leak, leading to headache in the days after placement. In two cases we have returned to the operating room several days later to resolve this problem with a fluoroscopically guided epidural blood patch. The introduction of spinal analgesic medication and the corresponding adjustment of systemic analgesics require careful attention because of the potential for oversedation and even respiratory depression. Because the intensity of afferent nociceptive transmission is diminished by the spinal medications, the stimulating effect of this nociceptive activity on alertness and respiratory drive is also diminished.
Occasionally in patients who have a tumor that involves a single peripheral nerve or nerve plexus, indwelling tunneled catheters can be placed percutaneously along the nerve or in the plexus sheath. Our preference is to use a combination of ultrasound and nerve stimulation guidance. These peripheral and plexus catheters can be extremely helpful if the pain remains highly localized. In a majority of cases, however, the tumor spreads beyond these more limited distributions, and we have therefore generally favored intraspinal infusions because of greater versatility in covering pain arising from a broader area. For similar reasons, our general preference is to choose intraspinal drug infusions rather than neurolytic nerve blocks except in very restricted circumstances, such as the use of a neurolytic celiac plexus blockade for a tumor that is almost entirely restricted to the upper abdominal solid viscera. Celiac plexus blockade has a good track record for treatment of refractory pain due to pancreatic cancer in adults. It affects visceral innervation only and produces no somatic sensory or motor deficits. Adverse effects such as diarrhea or orthostatic hypotension tend to be relatively short-lived and tolerable. For children and adolescents, our preference is to perform this procedure with use of general anesthesia with the patient in the prone position, using alcohol as the neurolytic agent. We use a posterior approach with computed tomography (CT) guidance, in collaboration with an interventional radiologist. Interventional approaches for pain in children were recently reviewed.
Psychological and Nonpharmacologic Approaches to Pain
Because of the multifactorial nature of the experience of pain, a multidisciplinary approach is often helpful in optimizing pain management. With use of psychosocial assessment it may be possible to identify and address factors that contribute to pain that analgesic interventions alone cannot address.
Depending on the developmental capacity of the child, interventions such as preparatory play, distraction, imagery, relaxation, deep breathing, hypnosis, and behavioral management can reduce anxiety and fear associated with pain. A child life specialist, psychologist, complementary medicine practitioner, or other specialist may be able to provide some of these interventions.
A variety of cognitive and behavioral approaches to pain and invasive procedures may be helpful to reduce pain and distress experienced by the child. In a study of 56 children aged 3 to 13 years, delivery of a cognitive-behavioral intervention was compared with preprocedure administration of diazepam or cartoon watching prior to bone marrow aspiration. The cognitive-behavioral intervention consisted of breathing exercises, positive reinforcement, and imagery or distraction to provide the child with imaginative and cognitive tools to cope with procedural stress. Overall, children who received the cognitive-behavioral intervention had lower pulse rates and distress and pain scores when compared with children who either received diazepam or watched cartoons. However, during the actual aspiration procedure, no difference was found between groups, suggesting that it did not ameliorate distress associated with the intense experience of the actual procedure. Cognitive behavioral therapy (CBT) does provide children with mechanisms for coping with distressing procedures and may explain why children who receive CBT display less distress after the procedure compared with children who receive an anesthestic via a mask but do not receive CBT.
Hypnotherapy can be performed in children as young as 4 years. Hypnosis can reduce procedural pain (e.g., from a bone marrow aspirate) and postoperative pain in children, as well as anticipatory nausea and dyspnea. In a randomized controlled trial of 30 children (ages 5 to 15 years) with cancer who were undergoing bone marrow aspiration, children who received either hypnosis or CBT reported less pain and anxiety compared with their baseline symptoms or control subjects who did not receive either intervention. Children who underwent hypnosis also demonstrated less procedure-related distress than did those in the other two groups.
Music, art, and play therapy may also be helpful in reducing anxiety associated with procedures. For example, soft lullaby music reduces heart rates in children undergoing the application or removal of a cast. Even when specific nonpharmacologic techniques are not employed, some behavioral strategies should always be used, including appropriately preparing the child, giving the child choices when possible, providing developmentally appropriate and honest explanations, and providing positive reinforcement. Patient education is also an important component of addressing pain.
Many patients find integrative therapies (i.e., therapies complementary to traditional medical approaches) to be of benefit in coping with stress, reducing the effects of treatment and illness, providing a sense of control, and enhancing quality of life. In a survey of adults participating in cancer clinical trials, 63% used at least one type of integrative therapy, with an average use of two therapies per patient. Few clinicians are aware of these practices and thus should routinely inquire about them.
Although many practices such as acupuncture, massage, and healing touch seem promising in reducing cancer-related pain, the evidence base supporting these therapies is weak. Barriers to the publication of such trials include issues of study quality and design such as small study size, high attrition rates, and lack of a comparison arm, particularly in fields where the placebo effect may be high. Studies with adequate power and duration and sham controls are needed to evaluate the efficacy of these interventions for cancer-related pain.
Pediatric Cancer Pain Syndromes
Although pain should be approached with a systematic approach and the use of analgesics, including opioids and adjuvants, certain cancer-related pain syndromes may respond to particular treatments.
Tumor-Mediated Pain
GI obstruction may cause pain. Management of GI obstruction is discussed in the section entitled “Other Gastrointestinal Symptoms.” Visceromegaly or invasion of organs, which often presents as a poorly localized, dull pain, is best treated with opioids, radiation, nerve blockade, or epidural/intrathecal techniques. Involvement of nerve or bone may result in neuropathic or bone pain, which is discussed later. Liver capsular pain may be alleviated by steroids. Elevated intracranial pressure may present as headache and vomiting and may respond to steroids or surgical decompression. In situations in which inflammation is thought to contribute to pain, the pain may respond to steroids or NSAIDs.
Pain Related to Administration of Antineoplastic Therapy
Antineoplastic therapy may produce a variety of pain syndromes. Injection of chemotherapy into a peripheral vein may cause local transient pain or phlebitis. Extravasation of a vesicant such as vincristine or anthracycline may cause local pain, burning, swelling, and/or redness, as well as tissue necrosis. These symptoms are most likely to occur when the agent is injected peripherally. Preclinical data showed that dexrazoxane, a reversible topoisomerase II catalytic inhibitor, reduced the number, severity, and duration of wounds that developed in a dose-dependent manner after extravasation. In a single-arm, open-label study of 53 humans with anthracycline extravasation, dexrazoxane was well tolerated. In this uncontrolled trial, one patient required surgical resection (1.8%).
Intrathecal chemotherapy may cause arachnoiditis, which is associated with fever, headache, nuchal rigidity, nausea, and vomiting. A new liposomal formulation of cytarabine appears to have an increased risk of arachnoiditis in children, which can be mitigated with systemic dexamethasone.
Prolonged steroid administration may result in avascular necrosis, which occurs most often in the hip. NSAIDs may help provide relief for this painful condition, although if the pain is severe, opioid therapy may be indicated. Pamidronate is a bisphosphonate that holds promise in reducing symptoms and increasing function in children who have painful osteonecrosis due to steroid therapy for acute lymphoblastic leukemia (ALL).
Bone Pain
Infiltration of bone is often experienced as a constant aching, which may be relieved by steroids, NSAIDs, or opioid analgesics. Isolated bone metastases may be treated with local treatments such as external beam radiation or surgical techniques such as vertebral body or long bone stabilization or other more invasive treatments for pain (see “Invasive Approaches to Pain Management”). Bisphosphonates, which reduce osteoclastic bone resorption, may be helpful when analgesics or radiotherapy are inadequate for the management of painful bone metastases. Diffuse bone metastases may also be treated with radioisotopes, but radioisotopes may cause myelosuppression.
Bone pain may also be caused by filgrastim or pegfilgrastim. Although the mechanism underlying such pain has not been clearly established, it is frequently attributed to filgrastim-stimulated marrow expansion. In a study of 100 adults receiving care in a community oncology practice, 79% experienced either moderate or severe bone pain due to use of pegfilgrastim. Pain was either diffuse or located in the lower extremities/hips or back. For persons with the most severe pain, the mean duration of pain was 2.8 days after the injection. NSAIDs provided relief for 74% of persons with moderate pain. Some persons with severe pain received opioids, but opioids provided relief in only 45% of patients. Interestingly, patients with lymphoma who were receiving concurrent steroids as lymphoma-directed therapy did not have significantly less bone pain.
Filgrastim-induced bone pain has not been well studied in children, although anecdotally it appears to occur less frequently in children than in adults. In a relatively small study of 28 children with cancer who received pegfilgrastim (100 µg/kg with a maximum dose of 6 mg for a total of 126 doses) after myelosuppressive chemotherapy, four children reported bone pain.
Although use of antihistamines to treat filgrastim-associated bone pain has been reported, no clinical trials have been performed to evaluate this practice. Until evidence indicates that antihistamines reduce this type of bone pain, antihistamines should not be used at the exclusion of established analgesic practices.
Mucositis
Mucositis is a common problem for children after chemotherapy, radiation, or bone marrow transplantation. It is particularly problematic in children undergoing high-dose chemotherapy or head and neck radiotherapy. Mucositis is associated with increased risk of infection and may impair intake of adequate nutrition. It usually self-resolves within 14 days or upon recovery of the bone marrow. The WHO has developed a mucositis rating scale that ranges from 0 (no visible evidence of mucositis, no pain, able to eat solids, and able to drink) to 4 (erythema, ulceration present, significant pain, and inability to eat or drink). Prophylactic antiviral or antifungal therapy does not decrease the incidence of mucositis. However, superinfection should be considered and cultures should be obtained when mucositis appears to be more severe or prolonged than anticipated.
Historically the mainstay of pain control for mucositis has been parenteral opioid therapy, usually via PCA. This mechanism of analgesia delivery is both safe and effective for this purpose. An adjuvant mouthwash containing diphenhydramine, 2% viscous lidocaine, and liquid antacid (e.g., Maalox) in a 1 : 1 : 1 formulation may be used in children older than 2 years who can spit out the medication rather than swallowing it. Topical application of straight viscous lidocaine carries the risk of systemic absorption if the mucositis is widespread. Although the true risk of aspiration due to local anesthesia of glottic structures is unclear, some centers avoid using this strategy because of this concern. A supersaturated calcium phosphate mouth rinse moistens and lubricates the oral cavity. Although results of studies of this agent have been mixed, the majority of studies support its use to reduce the severity/duration of mucositis and the pain associated with it. The Children’s Oncology Group is currently conducting a study of this rinse in preventing mucositis in children who are undergoing hematopoietic stem cell transplantation.
Other measures to alleviate mucositis include regular administration of oral sucralfate during chemotherapy, low-dose laser therapy, and gum chewing. Results of studies evaluating the efficacy of the free radical scavenger amifostine in reducing mucositis have been inconsistent. Severe mucositis can also cause abdominal pain, which is best treated with opioids. Perineal pain due to mucositis may also be addressed with a topical barrier cream to soothe and protect the area.
Recent advances in the understanding of the pathogenesis of mucositis have led to the development of targeted therapies. For example, palifermin is a keratinocyte growth factor that mediates epithelial cell growth and reduces epithelial cell apoptosis, factors that both appear to be important processes in the evolution of mucositis. Palifermin reduces mucosal injury as manifested by decreased mucositis duration and severity and a reduced need for analgesics and parenteral nutrition in adults undergoing high-dose chemotherapy and radiotherapy requiring peripheral blood stem cell rescue. In one study of adults undergoing high-dose therapy with autologous stem cell rescue, the incidence of WHO grade 4 mucositis was reduced from 62% to 20% ( P < .001).
The role of L-glutamine in mucositis has also been recently investigated. Depletion of L-glutamine is associated with epithelial damage, and increased levels are associated with healing. The role of uptake-enhanced L-glutamine suspension (AES-14) in reducing mucositis has recently been studied. In a placebo-controlled crossover study of 326 women undergoing chemotherapy for breast cancer, AES-14 reduced mucositis incidence and severity. In addition, AES-14 demonstrated a carryover effect, with patients receiving AES-14 before placebo having a lower risk of mucositis.
Neuropathic Pain
Neuropathic pain may occur from infiltration or compression of nerves, nerve damage from surgery, herpes zoster, or adverse effects from antineoplastic therapies such as vinca alkaloids, cisplatin, oxaliplatin, or bortezomib. Children often describe it as burning or shooting pain, and it may be accompanied by dysesthesia or hyperalgesia.
Neuropathic pain may be related to chronic pain states whereby chronic afferent input into the dorsal horn of the spinal cord leads to a hyperactive state. Mediators of this condition are excitatory amino acids, such as glutamate, which bind to the NMDA receptor. The NMDA/glutamate receptor is a calcium channel involved in opioid-resistant pain, neuropathic pain, allodynia, and hyperalgesia. NMDA receptor binding may lead to lasting increases in neuronal excitability, leading to persistence of neuronal hyperactivity. The end result of such a phenomenon is hyperalgesia, allodynia, spontaneous pain, and phantom limb pain, even after the painful stimulus has resolved. Methadone, with its NMDA receptor antagonist properties, may be the most appropriate opioid for neuropathic pain. However, no clear evidence from studies in humans shows that methadone is superior to other opioids in treating neuropathic pain. In addition, other approaches such as nerve blocks or adjuvant NMDA receptor antagonists may disrupt ongoing nociceptive input and neuronal hyperexcitability.
Most therapies for neuropathic pain have been evaluated in non–cancer-related neuropathic pain syndromes such as diabetic neuropathy. Medications for neuropathic pain include TCAs and the newer atypical antidepressants venlafaxine and duloxetine. TCAs often relieve neuropathic pain more quickly and at lower doses than are required to treat depression. TCAs reduce neuropathic pain by inhibiting serotonin and norepinephrine uptake, which increases transmission of inhibitory signals in the spinal cord. For children experiencing disturbed sleep, amitriptyline, a TCA that leads to greater sedation, may be a good choice. However, amitriptyline also has more anticholinergic effects such as xerostomia and constipation, in which case nortriptyline is an alternative TCA.
Duloxetine is the first antidepressant to be approved by the U.S. FDA for treatment of neuropathic pain, and in a recent multicenter randomized placebo-controlled crossover trial, it was demonstrated to reduce chemotherapy-induced painful peripheral neuropathy. Anticonvulsants such as topiramate, pregabalin, or gabapentin may also be effective. In the cancer population, gabapentin effectively reduced tumor-related neuropathic pain in a randomized controlled study of adults already receiving opioids, with lower average pain intensity scores and less dysesthesia in the gabapentin group. In some instances when tumors incite pain because of spinal cord compression, dexamethasone may reduce pain. Other interventions such as topical lidocaine or capsaicin, transcutaneous electrical nerve stimulation, surgical decompression, or nerve blocks may also relieve neuropathic pain.
Phantom Limb Pain
Phantom limb pain is a common phenomenon in children who undergo amputation of an extremity. It may be experienced as either stump pain or the type of pain experienced preoperatively, and it appears to improve over time. Phantom limb pain appears to be associated with preoperative limb pain. For this reason it is best approached with preemptive regional anesthesia. In our practice, regional anesthesia is used widely for anesthetic and analgesic management of amputations in children. The widespread use of ultrasound guidance has increased the reliability of continuous peripheral nerve blockade via indwelling catheters.
Additional measures to treat phantom limb pain include anticonvulsants, TCAs, and topical agents such as Lidoderm. Some small series have reported success with gabapentin and topiramate for phantom limb pain. A preliminary observational study found that use of a prosthesis to support limb use was associated with reduced phantom limb pain when compared with cosmetic prosthesis use. A few studies suggest that therapy with mirrors may relieve phantom limb pain, although the limitations of these studies, including small sample size, preclude definitive conclusions.
Pain Related to Graft-Versus-Host Disease
Graft-versus-host disease (GVHD) may cause a variety of pain syndromes. It is a common cause of abdominal pain in children undergoing bone marrow transplantation. Along with diarrhea, nausea/vomiting, weight loss, dysphagia, and early satiety, abdominal pain/cramping may occur in gut GVHD. Antimuscarinic/anticholinergic agents such as scopolamine may reduce colic from smooth muscle spasms. However, gut GVHD may be so severe that opioids are required for analgesia.
Oral GVHD usually does not cause pain. Development of oral pain is associated with ulceration or involvement of the soft palate. When evaluating pain in the setting of oral GVHD, one should also consider a concomitant infection such as herpes simplex virus or candidiasis. Oral GVHD pain is usually reduced with GVHD-directed treatment such as topical steroids or topical FK506 rinses. Pain associated with vulvovaginal GVHD may also respond to topical steroids or topical cyclosporine. Other types of GVHD, such as myositis or fasciitis, may require systemic analgesia.
Postoperative Pain
A broader discussion of treatment of postoperative pain in children and adolescents than that presented here can be found elsewhere.
Cancer surgery is often distressing and anxiety provoking for children and their parents. Unless specifically contraindicated, anxiolytic premedication with benzodiazepines should be considered routine, using oral or IV routes as appropriate. For patients who were treated with opioids on a regular basis before surgery, it is necessary to increase postoperative analgesic dosing accordingly. It is a common mistake to select routine parameters for opioid infusions or PCA for these patients, which invariably results in underdosing and inadequate analgesia.
For major tumor resections in the lower extremities, pelvis, abdomen, and thorax, we make extensive use of epidural infusions for postoperative analgesia. Epidural analgesia in children requires specific training regarding the technical aspects, as well as pediatric-specific management protocols and a level of acute pain service coverage that permits optimal dose adjustment. For patients receiving epidural infusions who were opioid tolerant before surgery, it may be necessary to provide a systemic opioid along with an epidural opioid postoperatively (despite a widespread “taboo” against this combination); alternately, larger than usual dosing of epidural opioids, the addition of epidural clonidine, or other approaches may be necessary to account for the patient’s preexisting opioid tolerance, which may be accompanied by hyperalgesia. Peripheral nerve and plexus catheters placed via ultrasound guidance are increasingly being used for surgery on the limbs. For thoracotomies, ultrasound-guided continuous paravertebral catheters are a promising alternative to thoracic epidural catheters.
Procedure-Related Pain
Brief procedures involving the use of needles, including venipunctures, IV cannulation, lumbar punctures, and bone marrow biopsies and aspirates, are a major component of cancer care and a major source of distress for patients. Approaches to management of these interventions should be individualized, based on consideration of the child’s age, temperament, coping style, and past pain experiences, as well as the intensity of the procedure (e.g., bone marrow biopsy is more painful than a simple venipuncture).
Explanations provided prior to procedures should be tailored according to the child’s age, developmental status, and coping style. The presence of the parents can be helpful, especially when the parents have been coached to help facilitate positive coping. Parents should be present to provide support, not to hold the child or assist the clinicians.
A variety of products are available to provide needle-free skin analgesia. These products include local anesthetic creams such as eutectic mixture of local anesthetics (EMLA), ELA-Max, tetracaine gel (Ametop), patches such as Synera, needleless injection systems such as Zingo, and lidocaine iontophoresis. These products differ in their onset time, propensity for vasodilation or vasoconstriction, cost, and availability in different countries. In a busy clinic, use of these topical anesthetic techniques is facilitated by standing order policies so they can be applied at an appropriate time before the planned procedure.
Several cognitive-behavioral approaches have been shown to be effective in reducing a child’s procedure-related distress, including hypnosis, guided imagery, and related approaches to achieving a relaxed or dissociated state. These approaches have the virtue of safety and generalizability. A patient who learns these techniques for one setting can apply them to other situations.
Conscious sedation, deep sedation, or brief general anesthesia can be very effective in alleviating the pain and distress of lumbar punctures and bone marrow biopsies and aspirates. The approach chosen will depend in part on the individual patient, the intensity of pain involved in the procedure, the degree of immobility required (e.g., for radiation therapy or magnetic resonance imaging), and available resources.
Considerable variation exists in the choices of drugs, the depth of sedation, and the personnel involved in different aspects of sedation. In some hospitals a two-tiered approach is taken, with lower risk patients receiving sedation administered by oncologists and nurses and higher risk patients cared for by pediatric anesthesiologists.
Other hospitals use a different two-tiered approach, with sedation for low- to intermediate-risk patients being administered by nurses and pediatric subspecialists from a sedation service who have specific expertise (e.g., pediatric intensivists or pediatric emergency physicians) and utilization of pediatric anesthesiologists to care for higher risk patients, those having more extensive procedures, or those requiring higher degrees of immobility. For example, for a cooperative, relatively healthy 12-year-old undergoing a lumbar puncture, a regimen that involves conscious sedation with midazolam and fentanyl and topical local anesthesia followed by local anesthetic infiltration is likely to be safe and effective. Conversely, a 2-year-old with a history of a very difficult to manage airway and cardiomyopathy who is undergoing bone marrow aspiration, biopsy, and a lumbar puncture would be at higher risk and therefore might be referred for management by a pediatric anesthesiologist.
In our view, regardless of local decisions about which specific specialists are involved in which procedures, pediatric oncologists need to advocate for sufficient institutional resources to ensure that effective and safe approaches to conscious sedation and general anesthesia are available for all children with cancer. Some general recommendations for sedation programs are listed in Box 71-1 .
- 1.
A standardized preprocedure history and physical should be performed to identify factors that might modify risk (e.g., airway anomalies, respiratory difficulties, and cardiac compromise).
- 2.
Use of procedure checklists should be encouraged.
- 3.
Nothing by mouth guidelines should be consistent with those recommended for children undergoing general anesthesia.
- 4.
Clinicians who are performing the procedure should have undivided attention during the procedure (e.g., their beeper and phone should be carried by someone else), and they should ensure that all necessary supplies (including kits, sterile supplies, chemotherapy, and tubes) are already in the room before bringing the child into the room or initiating sedation.
- 5.
Children receiving sedation should receive continuous monitoring, including a dedicated observer, continuous use of pulse oximetry, and regular recording of vital signs.
- 6.
All procedure locations should have at least the following supplies, equipment, or systems at hand:
- a.
Wall or tank oxygen supplies
- b.
Wall or portable suction devices
- c.
Monitoring equipment
- d.
Airway equipment, including bags and suitably sized masks, laryngoscopes, endotracheal tubes, oral airways, and laryngeal mask airways
- e.
An emergency cart, such as a code cart
- f.
Communication protocols (often assisted by a code button or phone system)
- a.
- 7.
After the procedure, children require observation until they meet standardized recovery criteria, and often for a period of time thereafter. During recovery, and especially when they no longer appear sedated, children remain at significant risk for injuries caused by falling, hitting their head on bed rails, and so forth.
- 8.
Observation and administration of medications should be performed by a clinician with no additional responsibilities—that is, not by the clinician performing the procedure. These clinicians should have training and competency in pediatric acute care and pediatric airway management.
- 9.
Clinics and hospitals performing pediatric sedation should track outcomes related to efficacy (from a child-centered viewpoint) and safety to facilitate system-based improvements in quality of care.
More detailed discussions of individual drugs and outcomes of pediatric sedation may be found elsewhere.
Reassessing Interventions for Pain
Careful reassessment to determine response to therapy is a key component in effectively addressing pain. In addition to reducing pain, interventions seek to reduce distress and functional impairment and enhance quality of life. Pain relief measures do not necessarily achieve all of these goals. For example, in one survey of adults with non–cancer-related pain, even when the degree of pain was controlled for, persons receiving opioid therapy did not have improved quality of life or improved function. Therefore in addition to evaluation of whether pain relief is attained, determination of whether increased functional capacity and increased quality of life is achieved is crucial in symptom management.
Barriers to Effective Pain Management
Increasing interest in pain and pain treatment in recent years has resulted in a better understanding of pathophysiologic mechanisms of pain, better treatment options, and greater availability of treatment for pain. However, these advances have not necessarily resulted in a reduced prevalence of pain in patients with cancer.
This paradox is due in part to the multitude of barriers to effective pain management. Clinician-related barriers include a focus on cure at the exclusion of attention to symptoms, lack of familiarity with pain management, fear of either regulatory repercussions for prescribing opioids or diversion of the medications to persons for whom the opioid is not prescribed, and underappreciation of the multifactorial nature of the experience of pain. Patient-related factors may include fear of addiction; fear of adverse effects, including respiratory depression; fear that treating pain with opioids is equivalent to “giving up”; and the misperception that opioids should be “saved” for severe pain.
Educational strategies for clinicians and families alike are likely to surmount many of these barriers to pain management. In addition, partnership with the child and family may enhance attention to this important issue and provide added opportunities for addressing barriers to treating pain adequately. For example, a randomized clinical trial tested the effectiveness of the PRO-SELF Pain Control Program, which actively involves adult patients and families in the pain management plan; findings included decreased pain intensity scores, an increased number of appropriate analgesic prescriptions, and increased analgesic intake in oncology outpatients who had pain from bone metastasis when compared with standard care. Such an approach is endorsed by the American Pain Society and is likely to contribute greatly to overcoming barriers to successful pain management.
Nausea and Vomiting
Pathophysiology
Vomiting may be caused by a wide variety of triggers, including motion, insult to the GI tract, hormonal changes, medications, and emotions. Vomiting is a complex process that involves both the central and peripheral nervous systems, with both biologic and psychological mechanisms playing a role.
During the past three decades strides have been made in understanding the pathophysiologic components of nausea and vomiting and in the development of agents aimed at them. Investigations have focused primarily on chemotherapy-induced nausea and vomiting (CINV). Prior to the 1990s, dopamine was the major neurotransmitter recognized as being important in mediating CINV, and dopamine blockade was the mainstay of therapy for CINV.
Vomiting is mediated by the vomiting center in the medullary lateral reticular formation. This center is not a discrete entity but instead is a group of loosely organized neurons in the medulla that are activated in a sequential manner during emetogenesis. Sources of afferent input to the nucleus tractus solitarius in the vomiting center include the chemoreceptor trigger zone (CTZ), the viscera (via vagal and sympathetic afferents), the midbrain, the vestibular system, and the cerebral cortex ( Fig. 71-3 ). Vomiting occurs when the vomiting center emits coordinated efferent impulses to several targets including the diaphragm, abdominal wall musculature, esophagus, and stomach.
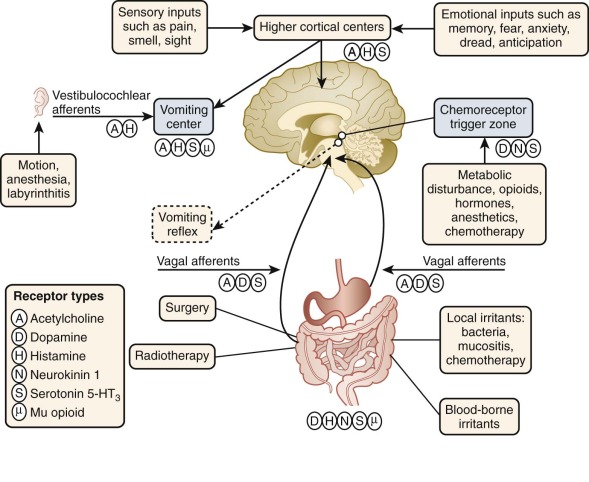
Chemotherapy-Induced Nausea and Vomiting
Chemotherapy can lead to nausea and vomiting through several mechanisms. One mechanism is by stimulation of the CTZ, which lies in the area postrema, in the floor of the fourth ventricle. Although the CTZ is part of the CNS, it contains chemoreceptors outside the blood-brain barrier that can detect emetogenic agents present in the circulation, as well as in the spinal fluid. However, evidence suggests that chemotherapy stimulates the CTZ indirectly because (1) it is unlikely that the CTZ has specific receptors that are activated by each type of chemotherapy agent; (2) the latency time to the onset of emesis is not compatible with direct action of the drugs on the CTZ; and (3) vagotomy and sympathectomy prevent cisplatin-induced emesis in the ferret, indicating that these inputs are needed for chemotherapy-induced vomiting to occur.
Chemotherapy can also stimulate the vomiting center directly. In addition, chemotherapeutic agents can stimulate the gut, leading to release of serotonin. This release in turn stimulates the vagus nerve, which activates the vomiting center, either directly or via impulses to the CTZ. The central cortex may also be involved in CINV by mediating emotions such as anxiety that play a role in the genesis of nausea.
Several neurotransmitter pathways are known to play a role in nausea and vomiting. Whereas histamine and muscarinic receptors are prominent in vomiting associated with motion sickness, serotonin, dopamine, and substance P appear to be particularly important in CINV. Central blockade of the D2 subtype of dopamine receptors in the area postrema and vomiting center is the mechanism of action of antidopaminergic antiemetics such as metoclopramide. It was later found that in addition to dopamine receptor antagonism, metoclopramide was an antagonist of the 5-hydroxytryptamine 3 (5-HT3) receptor, prompting interest in the role of this receptor in nausea and vomiting.
Serotonin is produced by enterochromaffin cells in the gut and is also found in multiple locations in the CNS. Several observations in both animals and humans confirmed that serotonin plays a major role in acute CINV. Cisplatin and cyclophosphamide-induced vomiting in the ferret was drastically reduced with the administration of intravenous, intraperitoneal, or intracerebral injection of serotonin S3 receptor antagonists. Serotonin receptor blockers and serotonin receptor blockade inhibited chemotherapy-induced vomiting in ferrets and in other animal systems. Depletion of serotonin stores was shown to also prevent cisplatin-induced vomiting.
In humans, studies by Cubeddu et al. demonstrated that strongly emetogenic regimens containing cisplatin or dacarbazine resulted in a marked increase in serotonin metabolites in plasma and urine with a time course similar to CINV symptoms in patients. In addition, treatment with a serotonin synthesis inhibitor inhibited both cisplatin-induced vomiting and increases in serotonin metabolites. The relationship between other chemotherapeutic agents and markers of serotonin metabolism has been further explored, with more emetogenic regimens resulting in higher serotonin metabolite levels. Chemotherapeutic agents appear to be toxic to the enterochromaffin cells lining the upper small intestine, resulting in generation of free radicals and release of serotonin.
5-HT3 receptors are present in the GI tract as well as vagal afferents. They are also found throughout the human brainstem, including a high concentration in the CTZ. Serotonin antagonists injected into the area postrema inhibit cisplatin-induced emesis in the ferret, but it appears that 5-HT3 receptor antagonists also exert their antiemetic effect at the level of the peripheral nervous system via serotonin blockade in the vagal nerve.
Substance P, which binds to the tachykinin neurokinin NK1 receptor, has more recently been implicated in mediating vomiting. Substance P is a neuropeptide found in both the CNS and in the GI tract. Because of its localization pattern and its ability to induce vomiting when administered intravenously, substance P was suspected to play a role in the genesis of vomiting. NK1 receptor antagonists appeared promising in that they inhibited vomiting induced by a range of agents known to produce vomiting by both central and peripheral mechanisms in the ferret. The site of antiemetic action of NK1 receptor antagonists has not yet been clearly defined. It may occur peripherally, at the level of vagal motor neurons that control relaxation of the gastric fundus. Investigation of the time course of the antiemetic effects of a 5-HT3 antagonist and the NK1 receptor antagonist aprepitant indicates that serotonin may mediate the early vomiting process (within the first 8 to 12 hours of cisplatin administration), whereas later vomiting may be mediated by the binding of substance P to NK1 receptors.
Classification of CINV
Acute and Delayed CINV.
Acute CINV, also known as posttreatment CINV, is traditionally defined as nausea and vomiting that occurs within the first 24 hours after administration of chemotherapy. Delayed CINV occurs 24 hours after chemotherapy and may last up to 5 days. Delayed CINV is typically associated with certain chemotherapeutic agents such as cisplatin and peaks 48 to 72 hours after administration of chemotherapy. The incidence of delayed CINV is as high as 89% in patients who receive cisplatin-based chemotherapy without antiemetic prophylaxis. The incidence of delayed CINV in patients who do receive antiemetic prophylaxis is as high as 73%.
Because not all acute CINV antiemetics are effective for delayed CINV and specific treatment for delayed symptoms may not be prescribed, delayed CINV may be more severe than acute CINV. The recent introduction of the NK1 receptor antagonist aprepitant has provided new options for controlling delayed CINV. However, it remains clear that the single best predictor of delayed CINV is acute CINV and that control of acute CINV is a key factor in preventing delayed CINV . For example, in a cohort of 705 patients receiving chemotherapy for the first time, when acute CINV was completely controlled with ondansetron plus dexamethasone, 92% had complete control of delayed CINV. However, of the patients who did have acute CINV, only 41% had complete control of delayed CINV.
Although some pharmacologic agents to control delayed CINV have been developed, lack of recognition of this problem remains a major barrier to its effective management. Grunberg et al. demonstrated that in a cohort of patients receiving moderately emetogenic chemotherapy for the first time, clinicians overestimated control of CINV, and this discrepancy was particularly notable for delayed CINV, in which case control of symptoms was overestimated by 21% to 28%. More than 75% of clinicians underestimated the incidence of delayed CINV. This misperception may in part explain the observation that fewer than half of persons who experience delayed CINV receive adequate prophylaxis that conforms to established guidelines. Given the extent to which delayed CINV negatively affects the lives of persons undergoing chemotherapy, improved practice, in conjunction with pharmacologic advances, is needed to reduce suffering from delayed CINV.
Relatively little information has been published regarding delayed vomiting in children. Pinkerton and colleagues found that of 27 children who received cisplatin, cyclophosphamide, or carboplatin and ondansetron, 9 (33%) experienced delayed emesis. In a larger prospective study of pediatric patients who did not receive antiemetics during the delayed phase after chemotherapy, two of six children (33%) who received highly emetogenic antineoplastics (cyclophosphamide, cisplatin, or carboplatin) and 12 of 110 children (11%) who received other chemotherapeutic agents experienced delayed vomiting, although the accuracy of the incidence of delayed vomiting in the “high risk” group is limited by the small number of children in that group. As has been appreciated in adult patients, children who vomited in the acute phase were significantly more likely to have delayed vomiting (45%) compared with children who did not experience significant acute vomiting (13%). In addition, chemotherapy lasting 2 or more consecutive days was associated with a significantly higher rate of delayed vomiting (39%) compared with chemotherapy administered on a single day (13%). In our experience, many children experience delayed CINV.
Anticipatory Nausea
Anticipatory nausea and vomiting is a conditioned response that occurs in anticipation of planned chemotherapy when vomiting has been poorly controlled in previous cycles. In adults, anticipatory nausea and vomiting occurs in one quarter of adults receiving chemotherapy and is associated with higher anxiety, a history of severe acute CINV, and a history of delayed CINV.
A similar pattern is seen in the pediatric population, with the severity of distress from nausea/vomiting, greater expectation for severe nausea/vomiting, and the severity of the nausea/vomiting that is actually sustained all corresponding with a higher likelihood of anticipatory CINV. Anticipatory nausea/vomiting is most likely to develop within the first 4 months of therapy, usually occurs hours before treatment, and is most severe at the actual time that chemotherapy is administered. Anticipatory nausea and vomiting in children appears to have features consistent with a conditioned response. These features suggest that this type of nausea and vomiting is a learned response, which explains why it is refractory to typical antiemetics.
Measurement and Assessment
Measurement.
Although nausea and vomiting are often lumped together, as in the term “CINV,” they may be regarded as two distinct phenomena. Nausea may be accompanied by physiologic changes such as pallor, sweating, or feeling hot or cold, but it is essentially a subjective experience that is measured by patient report. For this reason nausea cannot be studied in models other than humans. On the other hand, vomiting can be objectively measured. For this reason it is invariably used as the outcome of choice when evaluating interventions for nausea and vomiting. Complete control of CINV is a frequently used outcome and is defined as the absence of any vomiting for a defined period after administration of chemotherapy. That being said, vomiting does not always accompany nausea, and changes in vomiting do not necessarily reflect a concomitant change in nausea.
Although they can occur together, nausea occurs more frequently than does vomiting. Much attention has been focused on the prevention of vomiting, which is an important goal because previous uncontrolled vomiting may stimulate anticipatory vomiting in subsequent cycles of chemotherapy. However, nausea is also an important symptom to assess and control because it too impairs functioning and quality of life, even in the absence of vomiting. Finally, although advances such as the 5-HT3 receptor antagonists may have improved chemotherapy-associated vomiting, related posttreatment nausea has not improved. For all these reasons, attention to both nausea and vomiting is needed. Research that reports only vomiting as an outcome is a limited endeavor, and interpretations of assessments that rely solely on vomiting should be made with care.
Instruments have been developed to measure the subjective phenomenon of nausea in adults. Instruments to assess nausea in children, including children who are preverbal, have been described but are not in widespread use. One strategy for assessing nausea in children is to have them use a simple analogue or numeric scale to rate the severity of their nausea. It is known that children 5 years and older can reliably use a color analogue scale to report the severity of their pain and that children 7 years and older can report pain with a visual analogue scale or a numeric scale, and thus it is likely that they can report their nausea with similar reliability. A nausea scale of 0 to 10 with six faces (the Baxter Retching Faces BARF scale) for use in children ages 7 to 18 years who have nausea for a number of reasons has recently been demonstrated to have convergent and discriminant validity, as well as responsiveness.
Hinds et al. have shown that a symptom distress instrument that includes nausea assessment, which was designed for adults, is reliable and valid in adolescents with cancer. Other symptom distress scales that include nausea assessment, most notably the Memorial Symptom Assessment Scale, are also reliable and valid in children.
Within the pediatric population, parental report is also an important source of information when assessing nausea and vomiting. In one small study of CINV, parent-child dyads were found to have a moderate to strong association when reporting symptoms of nausea and vomiting. Studies by Zeltzer et al. have also showed a high degree of correlation between parental and child reports of nausea, although Tyc et al. have found that parents tend to underestimate their child’s nausea.
Assessment.
When nausea and vomiting in patients with cancer are considered, attention is often focused on CINV. When evaluating a patient with nausea/vomiting, however, it is imperative that other causes be considered in the differential diagnosis.
A full assessment of a patient with nausea and vomiting that extends beyond quantification of the severity of the symptom permits the generation of a complete differential diagnosis. Only in this way can all possible mechanisms and therefore all potentially efficacious interventions be entertained.
Features such as whether the nausea and vomiting is acute or chronic and intermittent or constant, along with whether it is associated with any particular factors, are important to consider. Other elements of the history, such as whether vomiting is projectile, bowel patterns, current medications, and prior history of nausea and vomiting, can also be helpful in delineating the cause and therefore finding potentially effective interventions. For example, obtaining a history that suggests constipation as the cause of nausea and vomiting will lead to a workup and treatment plan that is entirely different than if the cause is suspected to be chemotherapy or labyrinthitis.
Another important component of the history is an assessment of the impact of the symptom on the patient’s daily functioning and quality of life. Specific instruments to address these aspects have been developed, such as the Morrow Assessment of Nausea and Emesis, which is a self-report form that allows adults to assess their experience with acute, delayed, and anticipatory CINV.
Predictors of Nausea and Vomiting
The likelihood of CINV development and CINV severity is determined by both treatment-related and patient-related factors ( Box 71-2 ). The particular agent used is the primary treatment-related risk factor for CINV, but higher dosages of the agent, shorter infusion rates, combinations with other agents, and repeated cycles of chemotherapy can also increase the risk of CINV. When combination chemotherapy is given, antiemetics should be prescribed based on the most emetogenic chemotherapy drug and with the consideration that some combinations may potentially act synergistically in creating CINV.
- •
History of previous chemotherapy and prior chemotherapy-induced nausea and vomiting
- •
Prechemotherapy nausea
- •
Prechemotherapy anxiety
- •
Female sex
- •
History of motion sickness
- •
Low performance status
- •
Low social functioning
Patient-related factors, as determined in the adult population, also appear to affect the risk for developing CINV ( Box 71-2 ). In one multivariate analysis, low social functioning, prechemotherapy nausea, and female sex were found to be predictive of more severe CINV. Patients with a history of vomiting in a previous cycle are at greater risk of vomiting with the subsequent cycle in part, perhaps, because of anticipatory vomiting. Use of patient-reported outcomes from previous chemotherapy cycles is critical when prescribing antiemetics. An assessment of the intended treatment, as well as the patient’s individual risk factors, may help determine which patients are more at risk for the development of CINV and therefore in need of more intensive prophylaxis or rescue therapy.
Principles of Pharmacologic Therapy
Prevention of acute CINV is a key element in reducing delayed CINV. Because (1) the development of anticipatory CINV is based on prior experience, (2) severity of acute CINV predicts delayed CINV severity, and (3) rescue therapy is inadequate once nausea and vomiting have developed, the best approach to CINV and other situations in which nausea and vomiting may develop (e.g., in the postoperative period) is to prevent these symptoms from occurring in the first place. Although it is best to take a targeted approach based on the inciting cause and the suspected underlying pathophysiology and/or evidence demonstrating superiority of certain agents or classes in different situations, it is not always possible to know which agent to choose first. Guidelines for the initial choice of antiemetics, as well as dosing, are provided in Tables 71-2 and 71-3 . In this situation, use of one (or two) agents with titration of the dose and the addition of other agents if necessary might be the best strategy. For CINV, which is thought to be mediated through several pathways, a combination of antiemetics is usually warranted. This approach is particularly necessary with use of highly emetogenic chemotherapy, in which case monotherapy is almost always insufficient.
| Type of CINV | Prophylaxis | Breakthrough † |
|---|---|---|
| Acute CINV | ||
| High risk |
| Same as prophylaxis, initially as needed, then scheduled |
| Moderate or low risk |
| |
| Delayed CINV |
| Same as prophylaxis, initially as needed, then scheduled |
| Anticipatory CINV |
| Lorazepam |
- •
Choose appropriate prophylactic antiemetics based on emesis risk, determined by patient-related risk factors and the emetogenicity of the chemotherapeutic agent(s) being used.
- •
Assess the effect of the antiemetic regimen regularly.
† If breakthrough CINV occurs, strategies may be to (1) add a new agent, (2) change the dose or schedule of antiemetics in use but not already maximized, or (3) change administration of existing “as-needed” antiemetics to scheduled administration. In addition, the prophylaxis regimen for subsequent chemotherapy cycles should be augmented.
| Class | Dose and Route | Indication | Notes |
|---|---|---|---|
| 5-HT3 Receptor Antagonists | |||
| Ondansetron | 0.15 mg/kg dose PO every 8 h or 0.45 mg/kg/dose PO every 24 h, not to exceed 24 mg | First-line therapy for prophylaxis of high-, moderate-, or low-risk CINV or breakthrough CINV | May cause constipation and/or headache; available as an oral disintegrating tablet (that contains phenylalanine) or may be extemporaneously prepared |
| 0.15 mg/kg (not to exceed 8 mg/dose) IV every 8 h; no single dose should exceed 16 mg due to risk of QT prolongation | For patients with electrolyte abnormalities, congestive heart failure, bradyarrhythmias, or patients taking other medications with the potential to cause QT prolongation, consider ECG monitoring | ||
| Granisetron | <50 kg: 20 µg/kg IV/PO daily; maximum dose 1 mg ≥50 kg: 1 mg IV/PO daily | First-line prophylaxis for high-, moderate-, or low-risk CINV or breakthrough CINV | Available as an oral solution For patients with electrolyte abnormalities, congestive heart failure, bradyarrhythmias, or patients taking other medications with the potential to cause QT prolongation, consider ECG monitoring |
| Dolasetron | ≥2 yr: 1.8 mg/kg IV or PO as a single dose; maximum single dose 100 mg | May be extemporaneously prepared For patients with electrolyte abnormalities, congestive heart failure, bradyarrhythmias, or patients taking other medications with the potential to cause QT prolongation, consider ECG monitoring | |
| Dopamine Antagonist | |||
| Metoclopramide | 0.5 mg/kg IV or PO daily (for prophylaxis) 0.5 mg/kg IV or PO every 6 h (for breakthrough CINV) 1 mg/kg IV or PO every 6 h (for severe breakthrough CINV or if lower dose is ineffective) | High-risk prophylaxis; breakthrough CINV | High-dose metoclopramide: Prescribe with diphenhydramine and continue diphenhydramine for 24 h after the last dose to prevent EPS; if the metoclopramide dose is ≤1 mg/kg and the patient weighs >40 kg, scopolamine may be considered Treat EPS with diphenhydramine or benztropine |
| Corticosteroid | |||
| Dexamethasone | ≤BSA 1 m 2 : 10 mg/m 2 IV or PO daily BSA >1 m 2 : 10-12 mg IV or PO daily; may use 20 mg as a single dose on day 1 | High-risk prophylaxis for acute or delayed CINV; breakthrough CINV | Contraindicated in patients receiving pulmonary radiation therapy Consider lower dose if given with an NK-1 receptor antagonist |
| NK1 Antagonist | |||
| Aprepitant | ≥45kg: 125 mg PO ×1 on day 1, then 80 mg PO daily on days 2 and 3 | Prophylaxis of acute or delayed CINV | Limited data in pediatrics CYP3A4 substrate and inhibitor of metabolism of drugs that are CYP3A4 substrates including chemotherapeutic drugs (such as vincristine, irinotecan, imatinib, among others) and nonchemotherapeutic drugs (e.g., corticosteroids) |
| Anticholinergic | |||
| Scopolamine | 1.5-mg patch applied transdermally; change every 72 h | High-risk prophylaxis; breakthrough CINV | Use only if >40 kg (the patch cannot be cut); may cause dry mouth and/or blurred vision |
| Cannabinoid | |||
| Dronabinol | 2.5-5 mg/m 2 /dose PO every 6 h (10 mg/dose maximum) | High-risk prophylaxis; breakthrough CINV | Do not use in children <6 yr; use with caution if 6-12 yr; may cause confusion, ataxia, hallucinations; no IV form |
| Anxiolytic | |||
| Lorazepam | <20 kg: 0.025 mg/kg IV or PO every 6 h 20-40 kg: 0.0125 mg/kg IV or PO every 6 h >40 kg: 0.5-2 mg IV or PO every 6 h | High-risk prophylaxis; breakthrough; anticipatory CINV | May cause confusion, sedation, hallucinations, or memory impairment; avoid use of dose greater than 0.5 mg unless proven tolerance of 0.5 mg |
Drugs used to manage CINV include true antiemetics and adjuvants. Adjuvant medications may be used to allay anxiety, induce amnesia, or induce sleep. Although many of these agents have been investigated in adults, evidence regarding their use in children, including optimal dosing and potential adverse effects, is frequently much more limited. When evidence regarding dosing in the pediatric population is available, it is presented in this chapter. However, in many circumstances we must rely on evidence from adult studies and extrapolate appropriate dosing. Furthermore, guidelines and standard practices in adults may involve formulations of medications that cannot be adjusted for pediatric patients (e.g., capsules or patches). Use of medications in children that are only available in tablet or capsule form also may not be feasible for developmental or psychological reasons.
Dopamine Receptor Antagonists
Dopamine receptor antagonists include the butyrophenones (e.g., droperidol), phenothiazines (e.g., prochlorperazine), and substituted benzamides (e.g., metoclopramide; refer to the “Metoclopramide” section later in this chapter). Until the advent of 5-HT3 receptor antagonists and other antiemetics in the 1990s, dopamine-blocking agents were widely used. Use and efficacy of these agents are limited by their adverse effects, which include sedation and extrapyramidal symptoms (EPS). Children may be at higher risk for EPS, although EPS can often be prevented by concomitant administration of an anticholinergic agent such as diphenhydramine. Scopolamine, an antiemetic in its own right, is sometimes also used for this purpose. We have experience using scopolamine for EPS prophylaxis when the metoclopramide dose is less than or equal to 1 mg/kg/day and the patient weighs 40 kg or more.
Metopimazine, a phenothiazine derivative, is used as an adjunct to 5-HT3 antagonists to treat CINV. Although it is not marketed in North America, metopimazine is used for children in Europe, and a recent small study demonstrated improved emetic control when it was combined with ondansetron compared with ondansetron monotherapy. Olanzapine, an atypical antipsychotic agent, has fewer extrapyramidal effects than do older agents. In small studies in which it is combined with other antiemetics for CINV prophylaxis, olanzapine showed promise in preventing or treating CINV. Chlorpromazine and droperidol both may cause prolongation of the QT interval, and droperidol has been associated with serious cardiac adverse events. Extreme caution, including electrocardiogram monitoring, should be exercised if these agents are used, particularly in patients at risk for arrhythmias. Today use of droperidol is usually limited to the operative period in patients who do not respond to other agents.
5-HT3 Receptor Antagonists
5-HT3 receptor antagonists became the mainstay of therapy in the prevention and treatment of acute CINV since their introduction in the 1990s, when they were shown to be superior to the majority of preexisting antiemetics such as prochlorperazine and metoclopramide, even when highly emetogenic chemotherapy such as cisplatin is administered.
Compared with other agents, the superiority of 5-HT3 receptor antagonists in preventing acute CINV in children has also been confirmed. The efficacy of these agents appears to be further enhanced by the addition of a corticosteroid. In one study of 33 children, ondansetron plus dexamethasone provided a complete response in 61% of patients, whereas ondansetron alone provided a complete response in only 21% of patients. Ondansetron has also been shown to reduce acute CINV compared with placebo when chemotherapy is delivered intrathecally.
First-generation agents in this class include ondansetron, dolasetron, and granisetron. Taken together, the data on these agents indicate that these agents are equally effective when given at equivalent doses. These agents also appear to be equivalent in children, including in situations involving high-dose therapy such as preparative regimens for hematopoietic stem cell transplant. In one small study it was found that, compared with tropisetron, ondansetron better controlled acute CINV resulting from use of moderately emetogenic agents. However, no difference was found between the two agents with regard to controlling acute CINV resulting from use of highly emetogenic agents.
5-HT3 antagonists are quite well tolerated by children, with the most common adverse effects reported being mild headache and constipation. 5-HT3 inhibitors, in conjunction with a corticosteroid, are now recommended as first-line therapy for children who receive moderately to highly emetogenic chemotherapy.
Efficacy and tolerability of daily administration of high-dose ondansetron (24 mg or 32 mg by mouth daily) has been compared with a smaller dose (8 mg by mouth) given twice daily in adults. A large dose given daily is well tolerated in adults. In a large multicenter, randomized trial, the 24-mg daily dose provided the highest complete control of nausea and vomiting. The superiority of more intensive dosing has been confirmed by other investigators. The efficacy of 32 mg compared with 24 mg has also been confirmed by Tsavaris. However, because of the risk for QT interval prolongation in the IV formulation, the 32-mg IV dose is no longer available, and single (daily) doses exceeding 24 mg are not recommended.
Oral and IV routes of administration have been shown to be equally effective in both adults and children. Ondansetron, dolasetron, and granisetron are all available in IV formulations and as an oral tablet. Ondansetron is available as an oral dissolving tablet and as an oral liquid; these two preparations are especially attractive in the pediatric population. Granisetron may be extemporaneously compounded by an apothecary. Successful administration of 16 mg of ondansetron daily as a suppository has been described in adults but not in children.
A second-generation 5-HT3 receptor antagonist, palonosetron, differs from its predecessors in its stronger affinity for the 5-HT3 receptor and its prolonged plasma half-life (four times that of the other 5-HT3 antagonists), qualities that may enhance its duration of action. In randomized trials in adults, single doses of palonosetron appear to be at least as good in the prevention of acute CINV as single doses of first-generation antagonists delivered 30 minutes before administration of moderately emetogenic chemotherapy. Furthermore, the single dose of long-lasting palonosetron provides better protection from delayed CINV. Because of these findings, palonosetron is recommended rather than first-generation 5-HT3 receptor antagonists for adults receiving highly or moderately emetogenic chemotherapy. In pediatric patients palonosetron may prove to be a useful agent in the prevention of acute CINV during multiple-day therapy (in which acute CINV is repeatedly induced) and for the prevention of delayed CINV. Although administration of palonosetron for the prevention of CINV in children has been described, further study of dosing, safety, and efficacy is needed before palonosetron can be recommended for children.
Corticosteroids
Corticosteroids are frequently used as antiemetics, although their mechanism of action is unclear. Dexamethasone is the best-studied corticosteroid and the primary one in use today, although success with methylprednisolone has been described. The typical dexamethasone dose used for adults is 10 to 20 mg per day. Our starting dose for children is 10 mg/m 2 , to a maximum of 10 mg/day. For persistent vomiting, this dose may be doubled to a maximum of 10 mg given twice a day. If administered with an NK1 receptor antagonist, the corticosteroid dose should be halved.
Dexamethasone provides moderate protection from CINV, including delayed CINV, when used alone. Dexamethasone has been shown to be as effective as metoclopramide for acute CINV in adults receiving moderately and highly emetogenic chemotherapy without the extrapyramidal effects of metoclopramide.
Dexamethasone is especially useful in preventing delayed CINV from cisplatin, cyclophosphamide, and doxorubicin and may be even more effective for this purpose when combined with metoclopramide. Dexamethasone is also very effective in potentiating the action of other antiemetics, including 5-HT3 inhibitors and metoclopramide.
In the pediatric population, dexamethasone combined with ondansetron provided significantly more protection from acute CINV that occurred as a result of highly emetogenic agents than did ondansetron monotherapy; 77% of children receiving ondansetron monotherapy had at least one episode of vomiting, compared with 39% who received ondansetron with dexamethasone.
The benefit of improved protection from CINV must be weighed against the risks of corticosteroids. Adverse effects of corticosteroids include metabolic effects, gastritis, insomnia, hypertension, immune dysregulation, impaired wound healing, and adverse psychiatric effects such as emotional lability and, more rarely, psychosis. In general, short courses of corticosteroids do not usually produce significant adverse effects and are often very well accepted and tolerated by patients. Potential beneficial effects of corticosteroids are preservation of appetite and promotion of energy.
Other potential risks include a decrease in action of a biologic response modifier or induction of radiation pneumonitis when a steroid is withdrawn from patients who have undergone lung radiation. Caution is advised if corticosteroids are used as antiemetics in conjunction with a treatment regimen that is associated with a high risk of infection or GI toxicity such as induction regimens for acute myelogenous leukemia. Concomitantly administered corticosteroids may further increase these risks. Concerns about the impact of steroids on the blood-brain barrier have led to a desire to avoid the use of steroids as an antiemetic in patients with brain tumors, although chemotherapy-specific data are lacking. The use of steroids may be prohibited when they are already part of a patient’s chemotherapeutic regimen.
Metoclopramide
Metoclopramide, a procainamide derivative, exerts antiemetic effects via both a central mechanism (dopamine receptor blockade in the CTZ) and a peripheral mechanism (promotion of gastric emptying). It has been recognized as an effective antiemetic for adults for the past two decades as monotherapy or in conjunction with dexamethasone, lorazepam, or both. Low-dose metoclopramide (i.e., 0.1 mg/kg/dose) is effective in treating postoperative nausea and in promoting gastric emptying.
At high doses, metoclopramide also serves as a serotonin receptor antagonist and provides better protection from CINV. For these reasons, metoclopramide is usually given at high doses for CINV. At such higher doses, however, patients—and, in particular, children—are at a greater risk for extrapyramidal adverse effects such as dystonia and akathisia. In a dose-related toxicity study in children, significant extrapyramidal toxicity was observed at doses of 2 mg/kg or higher, and for this reason, high-dose metoclopramide is given with diphenhydramine to decrease the risk of extrapyramidal adverse effects. Extrapyramidal adverse effects can occur up to 24 hours later in patients who receive multiple daily doses of metoclopramide, and thus diphenhydramine administration should continue until 24 hours after the last dose of metoclopramide. We have also had personal experience using transdermal scopolamine as an anticholinergic agent in patients receiving no more than 1 mg/kg/day of metoclopramide. Because of the association of tardive dyskinesia with high-dose or long-term administration of metoclopramide, the FDA issued a boxed warning about this risk in 2009.
Cannabinoids
Delta-9-tetrahydrocannabinol (THC) is the active ingredient in cannabis, or marijuana. THC was approved by the FDA in 1985 for the treatment of emesis. Synthetic THC, called dronabinol (Marinol), has since become available. It is formulated in sesame oil and is available as 2.5- or 5-mg gelatin capsules. A homologue of THC, nabilone, is also now available in the United States.
THC binds to the CB1 receptor found in the central and peripheral nervous systems, as well as the CB2 receptor, which is found in nonneural tissues. THC exerts its antiemetic effect as a receptor agonist, in contrast to other antiemetics, which typically serve as receptor antagonists. THC also interacts with dopaminergic, serotonin, monoaminergic, noradrenergic, and opioid systems, which are pathways that mediate both emesis and pain.
Sallan and colleagues demonstrated that in patients who have CINV that is not controlled by standard antiemetic therapy, THC provided better complete protection from CINV (in 36 of 79 subjects) than did prochlorperazine (in 16 of 78 subjects). Interestingly, patients younger than 20 years had a higher proportion of complete responses than did older patients. Persons in the THC group also had significantly higher food intake. Adverse effects from cannabinoid administration include sedation, mood alterations (such as euphoria and dysphoria), dizziness, hallucinations, and arterial hypotension. Because of these potential adverse effects, cannabinoids are not typically considered first-line agents for CINV prophylaxis but are added to a regimen if first-line agents are not adequate for prophylaxis or breakthrough CINV.
An advantage of cannabinoids is their utility in treating pain. They are known to bind to kappa and delta receptors and act synergistically with opioids, a feature that may be useful when treating children with both nausea and pain. In addition, they are frequently used to stimulate appetite in patients who are not experiencing nausea or vomiting per se.
NK1 Receptor Antagonists
Aprepitant and its prodrug, fosaprepitant, are the only approved NK1 receptor antagonists. NK1 receptor antagonists are particularly effective in preventing delayed CINV, a historically difficult symptom to prevent. A study was performed to compae cisplatin-naive patients who received granisetron plus dexamethasone on day 1 and either (1) aprepitant (then called L-754,030) on day 1 or (2) aprepitant on days 1 through 5 or (3) placebo on days 1 through 5. In the aprepitant arms, 93% and 94% of subjects had no acute vomiting, compared with 67% in the placebo arm who had no vomiting. Subjects in the aprepitant arms also experienced significantly better complete protection from delayed vomiting (82% and 78%) compared with subjects who received a placebo (33%). In addition, minimal or no nausea was noted in 49% and 48% of subjects in the aprepitant arms and in 25% of subjects who received a placebo.
Other studies have replicated these impressive findings in terms of control of delayed CINV, but they have not demonstrated the improved protection from acute CINV seen in the aforementioned study. For example, in a double-blind multicenter trial of 351 patients, aprepitant with dexamethasone provided no benefit in preventing acute emesis when compared with the combination of granisetron and dexamethasone; however, the addition of aprepitant to granisetron and dexamethasone more than doubled the efficacy of this regimen in preventing delayed vomiting.
Aprepitant is generally very well tolerated by adults and adolescents and is easily administered by mouth daily. In addition, it appears to decrease delayed cisplatin-induced CINV that has been established to be refractory to the combination of 5-HT3 antagonists and dexamethasone. The efficacy of aprepitant appears to be sustained over multiple cycles of chemotherapy.
Aprepitant is usually administered for 3 consecutive days, because administration on additional days has not been shown to provide better protection from CINV. A pilot study comparing 1-day versus 3-day administration of aprepitant in the prevention of acute and delayed CINV in patients who were receiving highly emetogenic chemotherapy demonstrated no significant difference between the regimens. Additional studies are needed to confirm the noninferiority of the single-day aprepitant regimen.
Studies of the efficacy and safety of aprepitant in children are rare and quite limited methodologically. Use of aprepitant in children as young as 11 years using adult dosing (125 mg on day 1 and 80 mg daily on days 2 and 4) has been reported. In the only published prospective trial of aprepitant in children, 46 children aged 11 to 19 years who received emetogenic chemotherapy along with ondansetron and dexamethasone for 4 days were randomly assigned to also receive aprepitant or placebo for 3 days. In this study, which was designed to evaluate pharmacokinetics and tolerability, it was found that aprepitant was well tolerated but that febrile neutropenia occurred more often in children who received aprepitant, although the overall infection rate was higher in the placebo group. The limited sample size may have contributed to these findings and prevented statistical significance in the assessment of efficacy. The study demonstrated that pharmacokinetics in adolescents is similar to that of adults, and it is therefore reasonable to administer the adult dose to this age group. Administration of a lower dose (80 mg/day for 3 days) to smaller children (<20 kg) has been described, but the efficacy of this regimen in preventing CINV was not reported.
Fosaprepitant is an IV prodrug of aprepitant that is approved for administration in adults as a one-time dose prior to the administration of chemotherapy. Administration of this one-time dose given with ondansetron and dexamethasone provides protection from acute and delayed CINV that is equivalent to a regimen of aprepitant, ondansetron, and dexamethasone. Fosaprepitant dosing in children is currently being studied.
Aprepitant is a CYP3A4 enzyme pathway substrate and inhibitor and therefore may alter levels of drugs metabolized via this pathway. Concomitant administration with aprepitant may raise the level of drugs such as dexamethasone, methylprednisolone, fentanyl, cyclophosphamide, thiotepa, vincristine, or etoposide. For this reason aprepitant should be prescribed with care to avoid potential interactions, and the patient should be monitored closely for such interactions. It has been suggested that when aprepitant is coadministered with dexamethasone for CINV prophylaxis, the dexamethasone dose should be decreased by 50%. Aprepitant does not appear to alter the pharmacokinetics of ondansetron or granisetron. Aprepitant should be used with caution in patients who take warfarin.
Other Agents
Lorazepam is often used as an adjuvant to true antiemetics because of its anxiolytic and amnestic properties. Early studies evaluating the addition of lorazepam found that patients reported less anxiety and preferred regimens that contained this additional agent, although it did cause more sedation. The acute antiemetic effect of several agents is enhanced when lorazepam is given concomitantly. Interestingly, the addition of lorazepam to regimens that contained dexamethasone as prophylaxis against delayed CINV from cisplatin appears to decrease delayed CINV as well.
Scopolamine is a muscarinic antagonist that is known to reduce motion sickness. It is inadequate as monotherapy for CINV, but in conjunction with other antiemetics such as metoclopramide and dexamethasone, it reduces cisplatin-induced CINV. Scopolamine is administered as a 1.5-mg transdermal patch applied behind the ear that releases 0.5 mg/day and must be changed every 3 days. Because the patch is only available in the 1.5-mg size and cannot be cut, we normally reserve it for children who weigh 40 kg or more. Adverse effects from scopolamine include dry mouth, blurry vision, and mydriasis from systemic effects. Mydriasis on the side ipsilateral to the patch from unintentional touching of the patch followed by rubbing of the eyes can also occur.
Antihistamines such as dimenhydramine, hydroxyzine, and diphenhydramine are effective in reducing the nausea associated with vertigo and motion sickness. For CINV management, diphenhydramine is often given in regimens containing high-dose metoclopramide to prevent extrapyramidal effects. Diphenhydramine does not, however, enhance the antiemetic effect of metoclopramide, and apart from preventing extrapyramidal effects, it should not be used for CINV management.
Olanzapine is an atypical antipsychotic agent that binds to several receptors, including dopamine, serotonin, and to a lesser extent histamine and muscarinic receptors. Because of its action at multiple receptor sites implicated in CINV, it may hold promise as a therapy for CINV. In a small study of 10 adult patients receiving moderately to highly emetogenic chemotherapy with olanzapine, palonosetron, and dexamethasone as antiemetic prophylaxis, 100% had complete protection from nausea and vomiting in the first 24 hours after chemotherapy, with 50% and 75% of patients protected from nausea and vomiting, respectively, on days 2 to 5. Olanzapine is consequently included in some guidelines for adults with CINV. No studies evaluating olanzapine for children with CINV have been published.
Gabapentin has also been evaluated for CINV in adults. One study showed a decrease in peak nausea scores for both acute and delayed CINV, indicating that this agent may also hold promise as a therapy for CINV.
Special Cases
Delayed Vomiting.
Until very recent years, steroids and metoclopramide were the primary agents used to control delayed vomiting, with modest protection from delayed CINV in 48% to 57% of adult patients. Some studies have demonstrated efficacy of 5-HT3 antagonists in preventing delayed CINV and providing better protection when compared with existing drugs such as prochlorperazine. However, the majority of studies have demonstrated that the addition of a 5-HT3 receptor antagonist to the standard therapy, dexamethasone, does not confer greater protection from delayed CINV. In a meta-analysis of the efficacy of 5-HT3 antagonists, these antagonists did not confer protection from delayed CINV significantly beyond that provided by dexamethasone monotherapy. When ondansetron plus dexamethasone was compared with metoclopramide plus dexamethasone in randomized controlled trials, 5-HT3 was equivocal at best.
In one pediatric study evaluating ondansetron for acute and delayed CINV after treatment with carboplatin, cisplatin, Adriamycin with cyclophosphamide, or ifosfamide, ondansetron prevented acute CINV in 87% of children. Its efficacy in preventing delayed CINV was not nearly as high; it prevented only 20% of children from experiencing nausea and 50% of children from vomiting after administration of cisplatin or ifosfamide. A reasonable approach would be to start with dexamethasone and metoclopramide. In children who do not tolerate or who do not respond to metoclopramide, a 5-HT3 antagonist such as ondansetron may then be tried as an alternative.
In adults, some of the best protection from delayed CINV is conferred by the combination of a single dose of palonosetron with three doses of aprepitant and concurrent dexamethasone. In one study, this regimen provided a complete response (no emesis and no rescue medication) for 88% of patients in the acute period and 78% during the delayed period. Evidence for the efficacy of palonosetron in preventing delayed CINV is limited to single-day administration regimens. Interest in consecutive daily dosing of palonosetron to cover multiday chemotherapy has been expressed. Multiple dosing of palonosetron has recently been approved for adults.
Numerous studies in adults have demonstrated the effectiveness of aprepitant for delayed CINV, as previously described. Because most studies of aprepitant have been in the setting of single-day emetogenic treatment, it can be difficult to know how best to utilize this drug in the setting of the multiday regimens common in pediatrics. It is our practice to give the drug for up to 5 days, which may or may not continue beyond the end of the emetogenic treatment.
Anticipatory Nausea.
Because anticipatory CINV it is a learned response, typical antiemetics are generally ineffective. The best strategy for preventing CINV is the prevention of CINV in previous courses of chemotherapy. Because anxiety plays a key role in anticipatory nausea, use of an anxiolytic such as lorazepam may be effective.
Breakthrough Emesis.
Breakthrough emesis is defined as vomiting that occurs in spite of optimal preventive therapy. Although no studies of breakthrough emesis have been performed and no widely accepted standards are available, a reasonable approach is (1) ensuring that the patient is receiving maximal doses of current antiemetics and (2) adding a rescue agent from a category different from those the patient is already receiving. Although breakthrough agents may be started on an as-needed basis, scheduled administration may be needed during the current or future cycles.
Radiation-Induced Nausea and Vomiting
Radiation-induced nausea and vomiting occurs acutely in more than 90% of adult patients who receive total body irradiation for bone marrow transplantation and within 30 to 60 minutes in 80% of adults who receive single high-dose/large-field hemibody irradiation. Radiation-induced nausea and vomiting may also occur within 2 to 3 weeks in about 50% of adults who receive fractionated radiotherapy to the abdomen. The incidence and severity of radiotherapy-induced nausea and vomiting is largely related to the location of the radiation field, as indicated in Table 71-4 . In a prospective study of adults undergoing radiotherapy, the two radiotherapy-related risk factors for nausea and vomiting were the site of irradiation and the field size, with significantly more vomiting in persons who received radiation to the upper abdomen and in those with a radiation field size greater than 400 cm 2 . The only patient-related factor was previous experience with cancer chemotherapy. Because radiation to the upper/mid hemibody results in an increase in circulating serotonin metabolites and because of the efficacy of 5HT-3 receptor antagonists in this setting, it has been proposed that serotonin mediates radiation-induced emesis.