Chapter 8
Surgery in the Gastrointestinal Tract
Danielle Petersen, Vanessa Shaw and Tracey Johnson
Congenital Malformations
Danielle Petersen and Vanessa Shaw
Introduction
There are a number of congenital malformations that require surgery during the neonatal period. These malformations affect the oesophagus, stomach, and small and large intestines. The type of feed and the method by which it is given will be governed by the area of gut affected, the surgery performed to correct the defect and the condition of the remaining gut.
Oesophageal atresia and tracheo-oesophageal fistula
Oesophageal atresia (OA) has a prevalence of 1 in 2500 to 1 in 4500 births [1, 2]. The oesophagus ends blindly in a pouch, resulting in a non-continuous route from the mouth to the stomach. At birth infants are unable to swallow saliva and are noted to have excessive salivation. Aspiration of this saliva causes choking and cyanotic attacks. Eighty-six per cent of infants with OA also have a distal tracheo-oesophageal fistula (TOF) where the proximal end of the distal oesophagus is confluent with the trachea [2] (Fig. 8.1). In this case any reflux of stomach contents will enter the trachea and hence the lungs. Isolated ‘pure’ OA, where there is no fistulous connection with the trachea, occurs in 7% of infants and the rarer presentation of a tracheo-oesophageal fistula without OA (‘H’ fistula) occurs in 4%. The aetiology of OA is unknown and different environmental factors have been suggested to play a role [2].
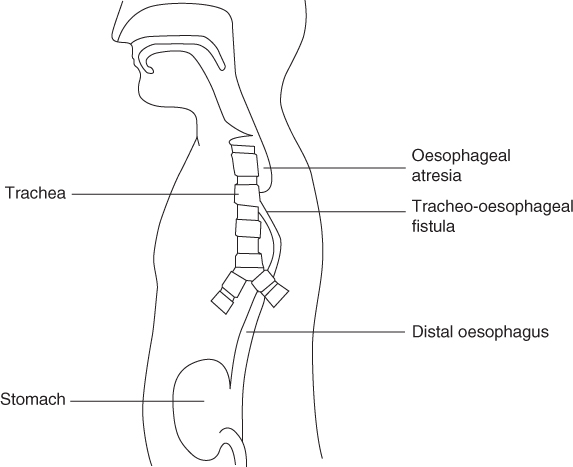
Figure 8.1 Oesophageal atresia and tracheo-oesophageal fistula.
OA is associated with other anomalies, associations or syndromes in about 50% of cases. Pedersen et al. [1] conducted a population based study using data from a large European database for the surveillance of congenital anomalies over 20 years: 1222 cases of OA were identified. The most common associated anomalies in these infants were congenital heart defects (29.4%), urinary tract anomalies (16.4%), other gastrointestinal anomalies (15.5%) and limb anomalies (13.1%). VACTERL association (vertebral, anorectal, cardiac, tracheo-oesophageal, renal and limb defects) occurred in 9.6% of the infants and CHARGE syndrome (coloboma, heart defects, choanal atresia, retarded growth and development, genital hypoplasia and ear abnormalities) in 1% of infants. Similar incidences are found in reports from other authors [2–4]. Over the last 50 years the mortality rate of infants with OA has decreased dramatically due to improvements in surgical, anaesthetic and neonatal care. Infants with a birth weight greater than 1500 g and with no major cardiac problems should have a near 100% survival rate. In those infants with a very low birth weight (<1500 g) or a major cardiac anomaly the survival rate is reduced to 82%, and in those with a very low birth weight and a major cardiac problem the survival rate is 50% [5, 6].
Following diagnosis, the upper pouch should be suctioned using a Replogle tube to reduce the risk of aspiration. The tube also helps increase the size of the pouch at the blind end of the upper oesophagus. Treatment of OA, whether associated with TOF or not, is undertaken as soon as possible after birth. It involves the repair of the oesophagus by anastomosing the upper and lower ends, after closing any TOF if present, so that both the oesophagus and trachea are separate and continuous. Until the lesion is corrected surgically, the infant cannot be fed orally. Depending on the length of the gap and the surgical management, either enteral or parenteral nutrition (PN) (p. 50) will be commenced [5].
Feeding the baby with oesophageal atresia and trachea-oesophageal atresia
A primary anastomosis is performed when the distance between the proximal and distal ends of the oesophagus is short enough for the two to be joined in one procedure. This is possible in the majority of patients, especially when a distal fistula is present. Most surgeons pass a trans-anastomotic tube (TAT) which allows gastric decompression in the early postoperative period and a route for nasogastric feeding.
Feeds are often started 48–72 hours after corrective surgery and may be given orally once the infant is swallowing saliva [2]. Ideally, breast feeding should be initiated or expressed breast milk (EBM) given. If this is not possible then a standard infant formula is used. Puntis et al. [7] found that 50% of infants undergoing a primary anastomosis were breast fed for a median period of 3 months. If a chest drain has been inserted postoperatively, nasogastric feeding via the TAT may be prolonged for 7 days or so until contrast studies show that the oesophagus is intact.
A study by Patel et al. [8] reviewed a 12 year period of a more simplified management of OA with TOF where chest drains, TAT and contrast studies were not routinely used. Seventeen infants were managed without a TAT and 23 with a TAT. The time to establishment of full oral feeding was 2–8 (average 3.9) days in the infants without a TAT, and 2–12 (average 5.9) days in those with a TAT. They concluded that sizeable minorities of infants do not require a TAT and that early introduction of oral feeds in this group is not associated with an increased risk of complications, such as developing strictures.
In some babies, the distance between the upper and lower ends of the oesophagus exceeds more than 3 vertebrae; this is termed ‘long gap OA’. An anastomosis between the upper and lower ends of the oesophagus is not possible and a delayed primary repair is required. The optimal surgical repair for babies with long gap OA remains controversial and there is no clear consensus, with practice differing between centres [5, 9]. In these infants the following interventions are suggested.
- Primary repair could be delayed for up to 12 weeks whilst maintaining suction to the upper pouch with a double lumen Replogle tube and feeding via a gastrostomy, allowing the gap to gradually shorten. Regular monitoring of the gap would be carried out and a repair attempted when the two ends can be approximated or overlap. If a TOF is present, it must be disconnected and the defect in the trachea closed before feeding can commence.
- Alternatively, tension can be applied to the oesophageal ends over a period of 6–10 days and a primary anastomosis is then performed.
- Another alternative is that the oesophagus is temporarily abandoned and a cervical oesophagostomy may be formed to allow the infant to swallow saliva and a gastrostomy placed for feeding [2]. In these infants the oesophagus is left for 3–6 months before attempting to join the upper and lower ends. Although cervical oesophagostomy prevents growth in the upper pouch of the oesophagus, the lower pouch hypertrophies and shortens the distance between the two ends.
If radiological studies show that it is still not possible to join the two ends of the oesophagus, the infant may be considered for an oesophageal substitution procedure. Feeding infants undergoing a delayed or staged repair presents more of a challenge than primary anastomosis.
The gastrostomy feed will be either EBM or infant formula and should be given at the same volume and frequency as the infant would receive had they been fed orally. Infants with cervical oesophagostomies should start sham feeding as soon as possible, which will allow the infant to experience normal oral behaviour. To facilitate normal development and coordination, the sham feed should be of the same volume as the gastrostomy feed, and the feed should be of the same duration and frequency so that the baby learns to associate sucking with hunger and satiety. Ideally the feed that is offered with sham feeding should have the same taste as that being put down the gastrostomy so that there is no refusal of feeds on the grounds of taste once the infant has an intact gut later. However, it is now more common practice for mothers who wish to give their babies breast milk to express their milk so that this can be given via the gastrostomy and the infant would be given infant formula by mouth for the sham feed. The sham feed seeps out of the oesophagostomy, along with saliva, and is usually managed by wrapping a towel or other absorbent material around the infant’s neck (Fig. 8.2). Puntis et al. [7] reported that 38% of infants with oesophagostomies were breast fed for a median duration of 2.5 months. There are, however, problems with sham feeding.
- It is difficult to coordinate holding the baby, feeding from a bottle and mopping up feed from the oesophagostomy while giving a gastrostomy feed. This event may defeat nursing staff let alone the mother coping single-handedly at home.
- One-third of infants with oesophageal atresia suffer from cardiovascular complications and may need ventilating, making sham feeding impossible.
- Infants may tire quickly and not be able to suck for long enough to take the same volume orally as is going through the gastrostomy.
- Many infants have small stomachs and initially require small volumes of gastrostomy feed frequently, e.g. 2 hourly, making it difficult to coordinate sham with gastrostomy feeding. However, this problem rapidly corrects itself when the feed volume is increased.
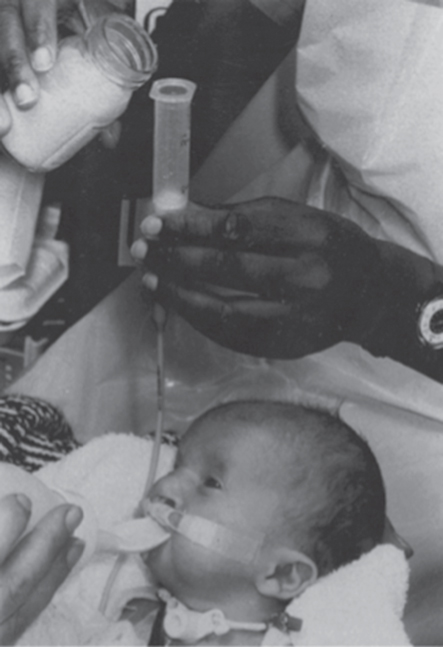
Figure 8.2 Infant with tracheo-oesophageal fistula and cervical oesophagostomy receiving oral sham feeds whilst being fed via a gastrostomy tube. The infant also has a tracheostomy and cleft palate and takes oral feeds from a Rosti bottle.
There is no route for sham feeding if an oesophagostomy has not been formed as part of the initial corrective surgical procedure. Infants deprived of oral feedings for the first weeks to months of life can experience difficulty in establishing sucking. This should not be a major problem if oral feeding is established within 2–3 months of life, but if oral feeding is delayed any longer than this it is associated with gagging and vomiting; the infant may avert its head at the very sight of the bottle or push out the teat with the tongue. Desensitisation to this oral aversion is a long, slow process. It is important to remember that feeding is not just a process of providing nutrition; infants are very alert at feeding time and develop cognitive and motor abilities while feeding.
Infants with oesophageal atresia with or without TOF can grow well on breast milk and normal infant formulas, if an adequate volume of feed is taken. If there is a problem with weight gain, feeds can be concentrated and supplemented or commercial nutrient dense formulas may be indicated (Table 1.18).
Feeding the older baby and toddler
In order to promote normal development, these babies should be weaned at the appropriate age of around 6 months. When the primary repair is performed soon after birth without complications, feeding is initiated early and normal feeding progression should be achieved. However, in infants with long gap OA, the primary anastomosis is most often delayed and these infants often experience feeding difficulties. It has been observed that weaning in infants that have undergone a primary anastomosis occurs around 6 months of age and the introduction of lumpy solids is delayed until 12 months of age [7]. If the infant is sham fed, weaning solids should also be sham fed if the oesophagus has not yet been joined up. There has been controversy in the past over what should go down the gastrostomy tube at this stage. A nutritionally adequate feed must be given in preference to weaning solids. In order to get strained weaning foods of the right consistency to go down the tube, they have to be watered down, thereby diluting their energy and nutrient content. If this practice is continued in the long term, failure to thrive could occur. In the study published by Spitz et al. [4] in 1987, of 148 children with OA, 27% of the patients were below the third centile for height and weight at 6 months and 5 years of age. A contribution to this could well have been the practice of inappropriate solids being administered down gastrostomy tubes, as was common practice at that time. A review in 2006 of 15 children with OA and TOF who had primary repair at birth found that all were between the 50th and 75th percentile of expected growth at 12 years of age [10]. A more recent review in 2012 of 57 children with OA and TOF found that 9% of patients were underweight and 16% were overweight, according to weight/height z scores [11].
If gagging is experienced when sham weaning solids are introduced, oral intake may be reduced to just tastes of food rather than giving large amounts in order to dispel the association between solid food and gagging. Foods need to be moist, and fibrous foods should be avoided. Fluid should be given at mealtimes to help the food go down. Both mother and child need to build up confidence about eating. Until joined up, sham feeding of age appropriate foods should continue orally with nutritionally adequate feeds via the gastrostomy. If the child does not have an oesophagostomy, enteral feeds should be given via the gastrostomy to promote normal growth. Standard infant formulas and high energy formulas (SMA High Energy, Infatrini or Similac High Energy) may safely be used up to the age of 1 year or so, but will need to be replaced by a more nutritionally adequate feed such as Paediasure, Nutrini or Frebini to maintain good growth in the older child (Table 3.2).
Oesophageal substitution procedures
There are various methods of correcting a long gap OA: gastric transposition, growth by traction, colonic interposition and jejunal interposition. Of these, the optimal technique remains controversial. However, there is consensus that the native oesophagus should be used wherever possible [9].
Colonic interposition involves removing a piece of the colon and transposing it into the chest between the oesophagus above and the stomach below. The advantages are that the required length of the graft is available and the diameter of the lumen of the transposed colon is appropriate for joining to the oesophagus. The disadvantages of this procedure are that the blood supply to the colon is poor; the transposed colon does not have very good peristaltic function to propel food down to the stomach; with time the transposed colon may lose its muscular activity; there is a high incidence of leakage around the anastomosis and the development of strictures.
In gastric transposition the whole stomach is mobilised and moved into the chest. The proximal end of the oesophagus is joined to the top of the stomach in the neck. The blood supply is excellent and the rate of both leakage and strictures is low. A review of gastric transposition by Spitz [12] in 192 children (138 had OA) showed that in over 90% of patients the outcome was considered good to excellent in terms of absence of swallowing difficulties or other gastrointestinal symptoms. There was a 4.6% mortality and 12% leak rate, with 20% of patients needing anastomotic dilatation for strictures. The procedure does have its disadvantages: poor gastric emptying; due to the bulk of the stomach being in the chest it reduces respiratory capacity; gastro-oesophageal reflux (GOR) can be a problem and dumping can occur (p. 150).
Foker et al. [13] reported outcomes in 70 infants with OA, with or without TOF, who underwent primary repair of their OA. Ten of these infants had long gaps of 5.0–6.8 cm of which four could not be pulled together initially and had traction sutures placed in the upper and lower segments. There were no surgery related deaths, but 11% mortality later in life. No discernible anastomotic leaks were recorded and one late recurrent TOF occurred; 46% of infants had significant GOR (34% of whom required a Nissen fundoplication) and 30% of patients needed dilatations due to anastomotic strictures. These symptoms were more common in infants with longer gaps. All patients over 2 years of age were reported to be eating satisfactorily or excellently and 93% of the children were eating like their siblings.
Ron et al. [9] conducted a survey of current practices of 88 paediatric surgeons based in the UK and abroad. The results showed that gastric transposition (48%) was the most preferred technique for long gap OA when primary anastomosis was not possible, and 94% of surgeons who used it were satisfied with it. Growth by traction was preferred by 39% of the surgeons, followed by colonic interposition by 8% and jejunal interposition by 5%.
Feeding post oesophageal substitution
The oesophagostomy, if present, is closed at the time of the oesophageal substitution. A feeding jejunostomy may be formed as a route for enteral nutrition while the child is sedated post surgery for gastric transposition, as the pre-existing gastrostomy can no longer be used. Gastrostomy feeding can continue if colonic or jejunal interposition has been performed. Oral nutrition is introduced as soon as possible, but supplementary overnight gastrostomy/jejunostomy feeds may be indicated until an adequate intake is taken by mouth. Oesophageal replacement procedures have their problems when feeding recommences as indicated above. The advantage of the gastric transposition is that there is only one anastomosis in the gastrointestinal tract, but the stomach is now sited in a much smaller place in the thorax than it usually occupies in the abdomen. The volume of feed or meals that can be taken comfortably may be greatly reduced, imposing a feeding regimen of little and often. The problem with colon interposition is that two areas of the gut have undergone surgery and anastomosis. Over time the transplanted colon makes a rather ‘baggy’ oesophagus because of the nature of its musculature, and the repaired oesophagus may not have normal peristaltic function. The colon may suffer temporary dysfunction because of surgical trauma and malabsorption may ensue, necessitating a change to a hydrolysed protein feed (Table 7.6). The end result of these surgical interventions may be oesophageal continuity, but not necessarily normal oesophageal function.
Problems with oesophageal function following repair
Gastro-oesophageal reflux is common following repair, with a reported incidence of 30%–45% [5, 11, 14, 15]. It may respond to medical management, e.g. thickening fluids (Table 7.26), positioning the baby appropriately after feeding or the administration of drugs such as metoclopromide and domperidone. These are dopamine receptor antagonists which stimulate gastric emptying and small intestinal transit, and enhance the strength of oesophageal sphincter contraction. H2 receptor antagonists such as ranitidine may be administered to reduce gastric acid output so that the reflux does less damage to the oesophageal mucosa. If GOR is severe and unresolved by these methods, surgical correction may be required by performing a fundoplication.
There are various anti-reflux operations, e.g. Nissen, Thal and Belsey. The choice depends on what the surgeon believes to be the best procedure for the individual child. The Nissen fundoplication is the most common and involves mobilising the fundus of the stomach and wrapping it around the lower oesophagus, thus fashioning a valve at the junction of the oesophagus and stomach. Between 30% and 39% of children undergoing repair for OA have significant GOR, leading to life threatening aspiration of feed, and require such anti-reflux surgery [11, 15]. Together with children with neurological dysfunction, infants and children who have a repaired OA and TOF comprise the majority of patients requiring such procedures.
The anti-reflux surgery is not without its postoperative complications. Whilst preventing reflux into the oesophagus, the fundoplication may also stop the child from burping, and gas bloat can be very uncomfortable in the stomach. Parents need to be taught to ‘wind’ their child through the gastrostomy tube. Although the child should not be able to vomit, they often experience severe retching which is very distressing for both child and parent, but this usually disappears after a few weeks or months. Fundoplication can also cause dumping (p. 150). Occasionally the valve created by the procedure weakens with time (usually a number of years, but sometimes after a few months) and GOR returns. If this cannot be managed medically the Nissen has to be ‘re-done’.
The change from receiving full nutrition from a gastrostomy/jejunostomy feed to maintaining an adequate intake orally is slow and there is often a long period where the child needs supplementary tube feeds while learning to eat normally. Prior to being joined up, the child has not experienced the sensation of a bolus of food passing the entire length of the oesophagus. Although the child may have been exposed to sham feeding, this method of feeding does not always lead to successful swallowing. Therefore, many children panic when offered any food other than in liquid form and the establishment of normal feeding has to proceed through stages of gradually altering the consistency of foods, from purées to finely minced and mashed foods and then to the normal diet.
After repair, whether the child has undergone a primary repair in the first few days of life, a delayed repair or a staged procedure, a circular scar will form where the upper and lower segments of the oesophagus are sutured together. With perfect healing the scar will have the same diameter as the oesophagus and will grow with the child. However, if the gap between the upper and lower pouch is >2.5 cm the two ends of the oesophagus will have been stretched to meet and the repair will be put under tension. This tension is thought to increase the risk of an anastomotic stricture developing. Other factors which have been suggested to influence stricture formation include a reduced blood supply in the lower oesophagus following repair, the type of suture material used and the suture pattern employed [5]. Legrand et al. [11] reported that of 57 patients with OA and TOF, 46% presented with anastomotic stenosis which required dilatation. These strictures prevent the normal passage of food with bread, meat, poultry, apple and raw vegetables being the foods most often cited as getting stuck.
If there is reflux of stomach contents up into the oesophagus, the acid will inflame the healing scar which may also lead to stricture formation. The presence of an oesophageal stricture has been reported in 52% of patients with GOR as opposed to 22% of patients with no reflux [16]. Children with these problems will often show difficulty in feeding and a reluctance to swallow; they will choke and splutter. Strictures require repeated dilatations to soften the scar tissue and allow the easier passage of solid food. The oesophagus may also go into spasm at the site of the anastomosis and particular foods like mince and peas may get stuck.
The frightening experience of repeated choking leads to fraught mealtimes that both parents and children come to dread. One-third of parents of babies with primary repair in Puntis et al
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree